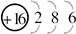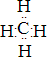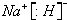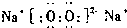��Ŀ����
��14�֣�A��B��X��Y��Z��ԭ���������ε����Ķ�����Ԫ�أ�����A��Yͬ���壬X��Zͬ���壬A��B��A��X�����γ�10�����ӻ����B��Z������������֮��Ϊ2�U3������������Y2X2��ˮ��Ӧ����X�ĵ��ʣ�����Һ��ʹ��̪��Һ��졣��ش��������⡣
(1) X�����ڱ��е�λ����_____________________________��
(2) ������Y2X2�ĵ���ʽΪ �������еĻ�ѧ�������� ������ţ���
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D�����
(3) A��X��A��Z�����γ�18�����ӵĻ���������ֻ��������Ӧ����Z�Ļ�ѧ����ʽΪ_____________________________________��
(4) A�ĵ�����X�ĵ��ʿ��Ƴ����͵Ļ�ѧ��Դ��KOH��Һ���������Һ���������缫���ɶ����̼�Ƴɣ�ͨ��������ɿ�϶���ݳ������ڵ缫����ŵ磬���缫��ӦʽΪ_____________________________________��
(5) д��������Y2X2��ˮ��Ӧ�����ӷ���ʽ____________________________��
(6) B�����������ĽṹʽΪ____________________________________��
(1)�ڶ����ڢ�A�� (2) A��C��ȫ�Ը��֣�
(3)H2O2+H2S=S��+2H2O (4)H2 �C 2e�� + 2OH����2H2O
(5)2Na2O2��2H2O��4 Na����4OH����O2�� (6) O=C=O
����:��������10���ӻ������о�������ԭ�ӣ����A��H��������Y2X2��ˮ��Ӧ����X�ĵ��ʣ�����Һ��ʹ��̪��Һ��죬�������ǹ������Ƶ����ʣ���X��O��Y��Na��B��ԭ������С��A�ģ����⻯����10���ӣ����Ըû������Ǽ����������B��C��N����ΪB��Z������������֮��Ϊ2��3������B�������ǵ�Ԫ�أ�ֻ����̼Ԫ�أ����Z��������������6������S��H��O��H��S�γɵ�18���ӵĻ�����ֱ�ˮH2O2��H2S��˫��ˮ���������ԣ�������л�ԭ�ԣ����߿��Է���������ԭ��Ӧ�������ʣ���ȼ�ϵ��������ʧȥ���ӣ�����������Ӧ������ڸ���ͨ�룬�缫��ӦʽΪH2 �C 2e�� + 2OH����2H2O��C�������������CO2������̼����֮���γɵ�̼��˫�������ԽṹʽΪO=C=O��