��Ŀ����
����Ŀ��(14 ��) ����ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�С�
��1��CrO3����ǿ�����ԣ������л����ƾ���ʱ�����ҷ�Ӧ�����Ż����ù������Ҵ������������ᣬCrO3����ԭ����ɫ�������[Cr2(SO4)3]����÷�Ӧ�Ļ�ѧ����ʽΪ��
��
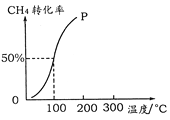
��2��CrO3�����ȶ��Խϲ����ʱ�ֽ⣬�������������¶ȵı仯����ͼ��ʾ��

��A ��ʱʣ�����ijɷ��� ���ѧʽ����B ��ʱʣ�����ijɷ��� ���ѧʽ��
���ӿ�ʼ���ȵ� 750Kʱ�ܷ�Ӧ����ʽΪ ��
��3��CrO3�� K2Cr2O7��������ˮ�����ǹ�ҵ����ɸ���Ⱦ����Ҫԭ������������֮һ�ǽ�����6�� Cr �ķ�ˮ��������ڣ�����������������������NaCl���е�⣺���������ɵ�Fe2+��Cr2O72��������Ӧ�����ɵ�Fe3+��Cr3+����������OH��������� Fe(OH)3 ��Cr(OH)3������ȥ[��֪ KspFe(OH)3��4.0��10��38��KspCr(OH)3��6.0��10��31]��
���������� NaCl ��������__________________________��
����֪�������Һ��c(Fe3+)Ϊ2.0��10��13 mol��L��1������Һ��c(Cr3+)Ϊ mol��L��1��
���𰸡�(14 ��)
��1��4CrO3��3C2H5OH��6H2SO4 =2Cr2(SO4)3��3CH3COOH��9H2O��3�֣�
��2����Cr3O8 ��2���� Cr2O3 ��2������ 4CrO3![]() 2Cr2O3��3O2����2����
2Cr2O3��3O2����2����
��3���� ��ǿ��Һ�ĵ�������2�֣���3��10��6 ��3�֣�
��������
������1�������������ṩ�ķ�Ӧ������������ʽΪ4CrO3��3C2H5OH��6H2SO4 =2Cr2(SO4)3��3CH3COOH��9H2O��
��2����CrO3�����ȶ��Խϲ����ʱ�ֽ�������ͼ����ʾ�����������A ��ʱʣ�����ijɷ���Cr3O8��B ��ʱʣ�����ijɷ���Cr2O3��
�����ȵ�750K��CrO3�ֽ��Cr2O3�����ܷ���ʽΪ4CrO3![]() 2Cr2O3��3O2����
2Cr2O3��3O2����
��3������������ NaCl ��������Ϊ����ǿ��Һ�ĵ�������
����֪ KspFe(OH)3��4.0��10��38��KspCr(OH)3��6.0��10��31����֪c(Fe3+)Ϊ2.0��10��13 mol��L��1����֪c(OH-)Ϊ2��10��25����c(Cr3+)3��10��6��

����Ŀ������ʵ�����������ͽ��۾���ȷ����( )
ѡ�� | ʵ����� | ʵ������ | ʵ����� |
A | ��ʢ��1 mLŨ������Թ��м���5 mL 0 .1 mol/L��K2 Cr2 O7��Һ | ��Һ�� ɫ���� | ����������Ũ�ȣ�ƽ��Cr2 O |
B | ��Mg(OH)2����Һ�м�����������茶��� | �����ܽ⣬��Һ����� | ˵����ӦMg2++2NH3��H2O |
C | ��ͬ�¶��£�ͬʱ���4mL0.1 mol/L. KMnO4)��������Һ�͢�4 mL 0.2 mol/LKMnO4��������Һ�У��ֱ����4mL 1 mol/L. H2 C2 O4��Һ | ������Һ����ɫ | ��ʵ�������£�KMnO4Ũ��ԽС����Ӧ����Խ�� |
D | ��ú¯�����ȵ�ú̿��������ˮ | ��������ɫ����ú̿ȼ�ո��� | ������ˮ��ʹú̿ȼ�շų���������� |
A. A B. B C. C D. D
����Ŀ����֪ij��ѧ��Ӧ��ƽ�ⳣ������ʽΪ![]() ���ڲ�ͬ���¶��¸÷�Ӧ��ƽ�ⳣ�����±���
���ڲ�ͬ���¶��¸÷�Ӧ��ƽ�ⳣ�����±���
t/�� | 700 | 800 | 830 | 1000 | 1200 |
K | 1.67 | 1.11 | 1.00 | 0.60 | 0.38 |
�����й���������ȷ���ǣ� ��
A. ����1 L���ܱ�������ͨ��CO2��H2��1 mol,5 min���¶����ߵ�830 �棬��ʱ���CO2Ϊ0.4 molʱ���÷�Ӧ�ﵽƽ��״̬
B. ������Ӧ������Ӧ�Ƿ��ȷ�Ӧ
C. �÷�Ӧ�Ļ�ѧ����ʽ��CO(g)��H2O(g) ![]() CO2(g)��H2 (g)
CO2(g)��H2 (g)
D. ��ƽ��Ũ�ȷ������й�ϵʽ��![]() �����ʱ���¶�Ϊ1000 ��
�����ʱ���¶�Ϊ1000 ��