��Ŀ����
����Ŀ���������С����ӹ�ҵ���������������ؼ��仯����Ӧ�ù㷺��
(1)��̬Gaԭ������_____��������ͬ�ĵ��ӣ���۵����Ų�ʽΪ_________��
(2)�������ڵ�����Ԫ���У���̬ԭ��δ�ɶԵ�����������ͬ��Ԫ����_______(��Ԫ�ط���)��
(3)������[(CH3)3Ga]���Ʊ��л��ػ�������м��塣
����700��ʱ��(CH3)3Ga��AsH3��Ӧ�õ�GaAs����ѧ����ʽΪ____________________��
��(CH3)3Ga��Gaԭ�ӵ��ӻ���ʽΪ__________��AsH3�Ŀռ乹����________________��
(4)GaF3���۵�Ϊ1000�棬GaC13���۵�Ϊ77.9�棬��ԭ����_______________________��
(5)�黯���ǰ뵼����ϣ��侧���ṹ��ͼ��ʾ��
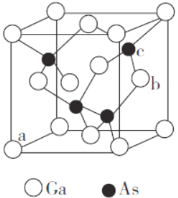
�پ�������Gaԭ�ӵȾ����������Asԭ���γɵĿռ乹��Ϊ_______��
��ԭ����������Ǿ����Ļ���Ҫ��֮һ����ʾ�����ڲ���ԭ�ӵ����λ�á�ͼ��a(0��0��0)��b(1��![]() )����cԭ�ӵ��������Ϊ______________��
)����cԭ�ӵ��������Ϊ______________��
���黯�ص�Ħ������ΪM g��mol-1��Ga��ԭ�Ӱ뾶Ϊp nm��������ܶ�Ϊ____g��cm-3��
���𰸡�8 4s24p1 K��Br (CH3)3Ga+AsH3![]() GaAs+3CH4 sp2 ������ GaF3�����Ӿ��壬GaCl3�Ƿ��Ӿ��� �������� ��
GaAs+3CH4 sp2 ������ GaF3�����Ӿ��壬GaCl3�Ƿ��Ӿ��� �������� ��![]() ��
��![]() ��
��![]() ��
�� ![]()
��������
(1)��̬Gaԭ�Ӻ�����31�����ӣ����������Ų�ʽΪ1s22s22p63s23p63d104s24p1����ͬ��ԭ�ӹ��������ͬ��
(2)��̬Gaԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s24p1��δ�ɶԵ�������1���������ڵ�����Ԫ���У���̬ԭ��δ�ɶԵ�����Ҳ��1�ģ�������Ų�ʽӦΪ1s22s22p63s23p64s1��1s22s22p63s23p63d104s1��
(3)����700��ʱ��(CH3)3Ga��AsH3��Ӧ�õ�GaAs�����������غ��֪��Ӧ��CH4�����ԭ���غ��д����Ӧ�Ļ�ѧ����ʽΪ��
�����ü۲���ӶԻ���ģ���жϷ��ӵĿռ乹�ͺ��ӻ���ʽ��
(4)GaF3���۵�Ϊ1000�棬GaC13���۵�Ϊ77.9�棬�����۵����ϴ�����������һ��Ϊ���Ӿ��壬һ��Ϊ���Ӿ��壻
(5)����GaAs�����У�����ÿ��Gaԭ�������Asԭ����4����
�ھ����о���a����Զ�ĺ���c��a������ߴ��ھ�����Խ����ϣ����ݼ���ԭ�����߾��������Խ��߳��ȵ�![]() ���ú�ɫ��c���������ƽ������Ϊ�����ⳤ��
���ú�ɫ��c���������ƽ������Ϊ�����ⳤ��![]() ��
��
�۾����к�Gaԭ����Ϊ8![]() +
+![]() =4��Asԭ����ĿΪ4�����к���GaAs����ĿΪ4������������Ϊ
=4��Asԭ����ĿΪ4�����к���GaAs����ĿΪ4������������Ϊ![]() g�����ݾ����Ľṹ��֪����������Խ��߳���ӦΪ������ԭ��ֱ���ij���֮�ͣ�����ԭ�Ӱ뾶Ϊ������Խ��ߵ�
g�����ݾ����Ľṹ��֪����������Խ��߳���ӦΪ������ԭ��ֱ���ij���֮�ͣ�����ԭ�Ӱ뾶Ϊ������Խ��ߵ�![]() �����߳�a=
�����߳�a=![]() ��10-7cm�����������Ϊa3=
��10-7cm�����������Ϊa3=![]() ��10-21cm3���ɴ˼��㾧�����ܶȡ�
��10-21cm3���ɴ˼��㾧�����ܶȡ�
(1)GaԪ�������ڱ��е�λ��Ϊ�������ڵ�IIIA�壬���������Ų�ʽΪ��1s22s22p63s23p63d104s24p1�����в�ͬ��ԭ�ӹ��������ͬ�������Ų��ڲ�ͬԭ�ӹ���ϣ����ӵ�����Ҳ�Dz�ͬ�ģ���˹���8�ֲ�ͬ�����ĵ��ӣ���۵����Ų�ʽΪ��4s24p1��
(2)��̬Gaԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s24p1��δ�ɶԵ�������1���������ڵ�����Ԫ���У���̬ԭ��δ�ɶԵ�����Ҳ��1�ģ�������Ų�ʽӦΪ1s22s22p63s23p64s1
(3)����700��ʱ��(CH3)3Ga��AsH3��Ӧ�õ�GaAs��CH4��������Ӧ�Ļ�ѧ����ʽΪ(CH3)3Ga+AsH3![]() GaAs+3CH4��
GaAs+3CH4��
�ڣ���CH3��3Ga��Ga�γ�3��������û�йµ��Ӷԣ�Ϊsp2�ӻ���AsH3����ԭ��As���Ӷ���=3+![]() =4��Ϊsp3�ӻ�����һ�Թµ��Ӷԣ��ʷ��ӿռ乹��Ϊ�����Σ�
=4��Ϊsp3�ӻ�����һ�Թµ��Ӷԣ��ʷ��ӿռ乹��Ϊ�����Σ�
(4)GaF3�������Ӿ��壬GaCl3���ڷ��Ӿ��壬�����۵����ϴ�
(5)���ɾ����ṹʾ��ͼ��֪���黯�صľ�������ʯ�ľ������ƣ�����ԭ�ӵ���λ����Ϊ4����Gaԭ����Χ��֮��������Ҿ�����ȵ�Asԭ����4�����γ���������ṹ��Ga����������������ģ�
�ھ����о���a����Զ�ĺ���c��a������ߴ��ھ�����Խ����ϣ����ݼ���ԭ�����߾��������Խ��߳��ȵ�![]() ���ú�ɫ��c���������ƽ������Ϊ�����ⳤ��
���ú�ɫ��c���������ƽ������Ϊ�����ⳤ��![]() �������������֪�����ⳤΪ1���ʸú�ɫ��c��������ƽ��ľ����Ϊ
�������������֪�����ⳤΪ1���ʸú�ɫ��c��������ƽ��ľ����Ϊ![]() ���ʸú�ɫ����������Ϊ ��
���ʸú�ɫ����������Ϊ ��![]() ��
��![]() ��
��![]() ����
����
�۾����к�Gaԭ����Ϊ8![]() +
+![]() =4��Asԭ����ĿΪ4�����к���GaAs����ĿΪ4������������Ϊ
=4��Asԭ����ĿΪ4�����к���GaAs����ĿΪ4������������Ϊ![]() g�����ݾ����Ľṹ��֪����������Խ��߳���ӦΪ������ԭ��ֱ���ij���֮�ͣ�����ԭ�Ӱ뾶Ϊ������Խ��ߵ�
g�����ݾ����Ľṹ��֪����������Խ��߳���ӦΪ������ԭ��ֱ���ij���֮�ͣ�����ԭ�Ӱ뾶Ϊ������Խ��ߵ�![]() �����߳�a=
�����߳�a=![]() ��10-7cm�����������Ϊa3=
��10-7cm�����������Ϊa3=![]() ��10-21cm3���ʾ������ܶ�
��10-21cm3���ʾ������ܶ� =
= g��cm-3=
g��cm-3=![]() g��cm-3��
g��cm-3��

����Ŀ����ѧ����ᡢ����������ء��������������ʵ�Ľ��Ͳ���ȷ����( )
ѡ�� | �������ʵ | ���ͻ��Ӧ�����ӷ���ʽ |
A | ���ȵĴ�����Һϴȥ���� | CO32��+H2O ��Һ�ʼ��ԣ��¶���������ǿ |
B | ������Ʒ�ڿ����з���pH��С | SO2+H2O=H2SO3 |
C | ����ĭ�������� | Al3+ + 3HCO3-= Al(OH)3��+3CO2�� |
D | �ü��ȷ���ȥNaCl�����л��е�NH4Cl���� | NH4Cl���������ȫ�ֽ��Ϊ�������ȥ |
A. A B. B C. C D. D