��Ŀ����
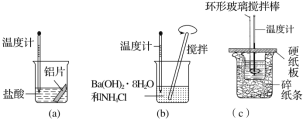
����Ŀ��ij����ѧϰС����ѧϰ��Na2O2��CO2�ķ�Ӧ����ΪNa2O2��SO2Ӧ��Ҳ���Է�Ӧ�������������ͼװ��![]() �г�װ������ȥ��װ�õ�����������
�г�װ������ȥ��װ�õ�����������![]() ����ʵ�飬̽��Na2O2��SO2��Ӧ�IJ���밴Ҫ��ش��������⡣
����ʵ�飬̽��Na2O2��SO2��Ӧ�IJ���밴Ҫ��ش��������⡣
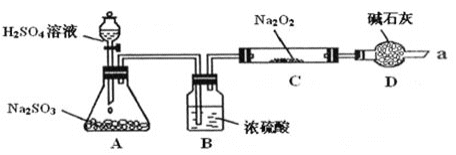
��д��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��_________
��װ��D�����ã����˿��Է�ֹ�����е�CO2��ˮ�����Ƚ���C����Na2O2��Ӧ��������__________��
����ͨ��������SO2��Na2O2��ַ�Ӧ�����Ƕ�C�й������������¼��裺
����1��ֻ��Na2SO3��
����2��_________��
����3������Na2SO3������Na2SO4��
a��Ϊ��һ��ȷ��C�з�Ӧ��������ijɷ�(Na2O2�ѷ�Ӧ��ȫ)����ͬѧ���������ʵ�飺

��ͬѧ�ɴ˵ó����ۣ�������Na2SO4���÷����Ƿ����__________![]() ����������������
����������������![]() ��������_______��
��������_______��
b��������2������д��SO2��Na2O2��Ӧ�Ļ�ѧ����ʽ��_________��
c����ͬѧ���������ʵ���һ��ȷ�ϲ���ijɷ֡�
ʵ�鲽�� | ���� |
��ȡ����C�й���������Թ��У���������������ˮ�ܽ⡣ | ����ȫ���ܽ� |
�������Թ��м��������ϡ���ᣬ�����ɵ�����ͨ����������KMnO4��Һ�С� | ����KMnO4��Һ��ɫ |
������ڷ�Ӧ����Թ��У�����������BaCl2��Һ�� | ������ɫ���� |
������н�����������ͨ����������KMnO4��Һ�У�������Ӧ�����ӷ���ʽΪ�� ________��ͨ������ʵ������ȷ������__________������(ѡ��1��2��3)��
���𰸡�Na2SO3 + H2SO4 = Na2SO4 + H2O+SO2�� ����β��(δ��Ӧ��ȫ��SO2����)����ֹ��Ⱦ���� ֻ��Na2SO4 �� ������ǿ�����ԣ������ܽ������ᱵ����Ϊ���ᱵ Na2O2 + SO2 = Na2SO4 2MnO4�� + 5SO2 + 2H2O = 5SO42��+ 2Mn2+ + 4H+ 3
��������
��װ��A���������������ᷴӦ��
��װ��D�������Ƿ�ֹ�����е�ˮ�����Ͷ�����̼����Cװ����Na2O2��Ӧ�����������չ�����SO2��������Ⱦ������
�Ƕ���������������Ʒ�Ӧ�ķ���ʽ����Ϊ��2Na2O2 +2 SO2 = 2Na2SO3+ O2����Ҳ�п���Ϊ��Na2O2 + SO2 = Na2SO4��a�����ɵİ�ɫ������������������ᱵ����������Ὣ�����ᱵ����Ϊ���ᱵ��b��������2����������������ԭ��Ӧ�õ�SO2��Na2O2�ķ�Ӧ��ѧ����ʽ��c������������л�ԭ�ԣ��ܹ������Ը��������Һ������д�����ӷ���ʽ���ٸ������������ó����ۡ�
��װ��A���������������ᷴӦ�Ļ�ѧ����ʽΪ��Na2SO3 + H2SO4 = Na2SO4 + H2O+SO2����
�ʴ�Ϊ��Na2SO3 + H2SO4 = Na2SO4 + H2O+SO2����
��װ��D�������Ƿ�ֹ�����е�ˮ�����Ͷ�����̼����Cװ����Na2O2��Ӧ�����������չ�����SO2��������Ⱦ�������ʴ�Ϊ������β��(δ��Ӧ��ȫ��SO2����)����ֹ��Ⱦ������
�Ƕ���������������Ʒ�Ӧ�ķ���ʽ����Ϊ��2Na2O2 +2 SO2 = 2Na2SO3+ O2����Ҳ�п���Ϊ��Na2O2 + SO2 = Na2SO4���������ֻ��Na2SO3�����ܼ���Na2SO3����Na2SO4��������ֻ�������ƣ����Լ���2Ϊ��ֻ��Na2SO4���ʴ�Ϊ��ֻ��Na2SO4��
a�����ɵİ�ɫ������������������ᱵ����������Ὣ�����ᱵ����Ϊ���ᱵ������ȷ��������Na2SO3����Na2SO4������У��ʲ��ܵó�����ֻ��Na2SO4�Ľ��ۣ��ʴ�Ϊ����������ǿ�����ԣ�������Խ�BaSO3��������ΪBaSO4��
b��������2������SO2��Na2O2��Ӧ�Ļ�ѧ����ʽΪ�� Na2O2 + SO2 = Na2SO4���ʴ�Ϊ��Na2O2 + SO2 = Na2SO4��
c������������л�ԭ�ԣ��ܹ������Ը��������Һ��������Ӧ�����ӷ���ʽΪ��2MnO4�� + 5SO2 + 2H2O = 5SO42��+ 2Mn2+ + 4H+��Ҫ��֤������Na2SO3��Na2SO4�����Լ���SO32����SO42����ʵ�鷽��Ϊ����ȡ����C�й���������Թ��У���������������ˮ�ܽ⣬������ȫ�ܽ⣻�������Թ��м��������ϡ���ᣬ������������ȫת���ɶ����������壬������������ͨ����������KMnO4��Һ�У����������Һ��ɫ��֤�������к����������ƣ�����������Ӧ����Թ��У�����������BaCl2��Һ��������ɫ�������ð�ɫ����Ϊ���ᱵ��˵��ԭ��Һ�к��������ƣ��Ӷ�֤������3�������ʴ�Ϊ��2MnO4�� + 5SO2 + 2H2O = 5SO42��+ 2Mn2+ + 4H+��3��

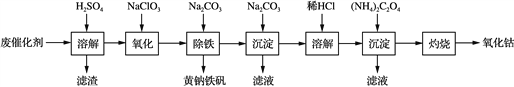
����Ŀ���Ƽ����������������̿ɽ���ת��Ϊ��п�����壺

��֪���ٻƼ�������������Ҫ��Fe2O3��ʽ���ڣ�п��Ҫ������п(ZnSO4)������п(ZnO)������п(ZnSiO3)��ʽ���ڣ��Ƽ���������ijЩԪ�سɷ����±���ʾ��
Ԫ�� | Fe | Zn | Cu | Cd | Ca | Mg | Si |
�������� | 28.9 | 8.77 | 0.37 | 0.18 | 0.37 | 0.84 | 4.63 |
��NH4F��Һ���ڳ���Mg2+��Ca2+����Fe��Cd�Ľ���������
���������������1����Ҫ�ɷ�Ϊ________(д��ѧʽ)��Ϊ����߽����ʣ��ɲ�ȡ�Ĵ�ʩ��________(д��һ�ּ���)��
������ԭ�����������У�����������Ϊ�˳�ȥ��Һ��________��________�Ƚ����������ӡ�
�Ǽ���(NH4)2S����Cd2+ʱӦ���������ԭ����________�����˹�������Һ����ʱ���������ᵼ��Cd2+ȥ����ƫ�ͣ�ԭ����________��(��֪��CdS���ܶȻ�Ksp = 8��10��27��FeS���ܶȻ�Ksp = 4��10��19��ZnS���ܶȻ�Ksp = 1.6��10��24)
��д����������������������FeCO3�����ӷ�Ӧ����ʽΪ��________��
����п��������һ����Ҫ�Ĵ��Բ��ϡ��ⶨ��������ZnO��ʵ�鲽�����£�

��д������(Mn2+)�����е����ӷ���ʽ________��
��ȷ��ȡ25.00 mL��ҺA���ڱ������ö��ӳ���ָʾ������0.0100mol/L��EDTA(Na2H2Y)����Һ�ζ����е�Zn2+ (��Ӧԭ��ΪZn2+ + H2Y2�� =ZnY 2�� + 2H+)�����ζ��յ�ʱ����EDTA����Һ20.00 mL��ͨ������ȷ������������ZnO����������Ϊ________��
����Ŀ����ͼ��ʾ����֤�������ʵ���ʵ�飬a��b��d��e�ǽ��������Һ����ֽ����KMnO4����μ�һ��Ũ�������������һ������������档��֪��2KMnO4��16HCl�D��2KCl��5Cl2����2MnCl2��8H2O
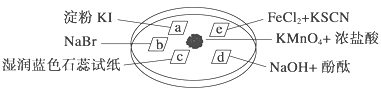
��ʵ������������ͻ��������ȷ���� (����)
ѡ�� | ʵ������ | ���ͻ���� |
A | a��������b�������ɫ | �����ԣ�Cl2>Br2>I2 |
B | c���ȱ�죬����ɫ | ������ˮ�������������� |
C | d��������ɫ | ������ˮ������Ư�������� |
D | e�����ɫ | ��ԭ�ԣ�Fe2��>Cl�� |
A. A B. B C. C D. D
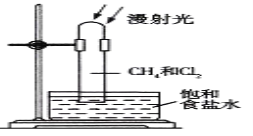
����Ŀ��ij����С���һ����̼��ԭ��������ʵ�������IJ������Ũ����Ȥ����ͨ��ʵ����̽����ɷ֡�
����ʵ��װ�ã�

��װ��B�з��������ӷ���ʽ�� ______��װ��B�������� ______��
����ʵ����������A�еķ�ĩ�ɺ�ɫ��Ϊ��ɫʱ��ֹͣ���ȣ�����ͨһ����̼����ȴ�����£�ֹͣͨ����ͬʱ�۲쵽�����ʯ��ˮ����ǡ�
����ʵ����ۣ�
����Ϊ����������ʵ����������жϳ����ɵĺ�ɫ����Ϊ��������
����Ϊ����������ʵ����������֤�����ɵĺ�ɫ����Ϊ��������������һ��ʵ�飺�ô���������ɫ���壬�����к�ɫ���屻�������������ǵó����ɵĺ�ɫ����Ϊ�������Ľ��ۡ�
����ͨ���÷�Ӧ��������϶����ǵĽ��������жϣ���ͨ��ʵ�����������ԣ�
����һ�������£�һ����̼���������ڼ��������£��ɷ������·�Ӧ��
CO +3Fe2O3 = 2Fe3O4+ CO2�� 4CO + Fe3O4 = Fe+ 4CO2��
������������(Fe3O4)Ϊ��ɫ���壬��ǿ���ԣ��ܹ�������������
�ס���ͬѧ�Ľ��� ______����Դ����۵������� ______��
����ʵ��̽��
�Է�Ӧ�����ɷ�������裺
����1����Ӧ�������ֻ��Fe��
����2����Ӧ�������ֻ��Fe3O4��
����3����Ӧ������� ______ ��
Ϊȷ��ʵ�����������еijɷ֣���ͬѧ�������ʵ�飬����������ѡ�Լ���������������ɸ�̽�����̣�������д�ڴ����Ӧλ�á�
��ѡ�Լ���������1mol/L CuSO4��0.01mol/L KSCN��Һ��1mol/L ���ᡢ0.01mol/L ��ˮ���Թܡ�����������ͷ�ιܡ�
ʵ����� | Ԥ������ͽ��� |
����һ��ȡӲ�ʲ������й�����������ֱ���A��B�Թ��У���������1mol/L CuSO4��Һ�������ܽ⡣ | ����A�Թ��к�ɫ���岻�ܽ⣬����û�й۲쵽�����������ɫ����Ϊ ______�� ����B�Թ����к�ɫ������������˵����ɫ�����к��� ______�� |
����������Թ�B����Һ���ˣ������ù���ϴ�Ӹɾ��������1mol/L����������ηֱ��������0.01mol/L��ˮ������0.01mol/L KSCN��Һ�� | ������Һ�����ɫ����______�� ������Һ���ɫ����______�� |
��������̽������ͬѧ��ͼͨ����Ӧǰ����������ı仯��ȷ����ɫ����ijɷ֣�����Ϊ������(�����������ڷ�Ӧ����ȫ��Ӧ)______(������������������)�������ǣ�______��