��Ŀ����
��10�֣��״���һ�ַdz��õ�ȼ�ϡ��۵�-97.8��C���е�64.5��C��һ�������£�
CO��H2��Ӧ���Ƶü״���CO+2H2 CH3OH
CH3OH
ͼ1��ʾ�÷�Ӧ���й����е������仯��

ͼ2��ʾ100��C�������Ϊ2L�ĺ��������м���4molH2��һ������CO��CO��CH3OH��g����Ũ����ʱ��仯�����
��1����֪CO��ȼ����Ϊ283kJ/mol��H2��ȼ����Ϊ285.8kJ/mol�����ͼ1д��Һ̬CH3OHȼ���ȵ��Ȼ�ѧ����ʽ ��
��2��������ͼ2���㣺���¶��£���ӦCO��g��+2H��g�� CH3OH��g����ƽ�ⳣ��Ϊ ��10min�������ڵ�ѹǿ��Ϊԭ���� �����ı����������������COת���ʵ��� ��
CH3OH��g����ƽ�ⳣ��Ϊ ��10min�������ڵ�ѹǿ��Ϊԭ���� �����ı����������������COת���ʵ��� ��
A�������¶� B���Ӵ���
C�����������ʹ��ϵѹǿ���� D���ٳ���1molCO��2molH2
E�����º��ݸ�Ϊ���º�ѹ
��3����֪��CH3OH������һ��������ת��ΪHCOOH��HCOOH��CH3COOH�������ơ�25��C��0.1mol/LHCOOH��Һ��pH>1�������£���0.1mol/L��HCOOH��Һ�еμ�NaOH��Һ������Һ������Ũ�ȹ�ϵ���㣺c��HCOO-��<c��Na+��ʱ����Ӧ���������Ϊ ��������ĸ��
A��NaOH���㣬HCOOHʣ��
B��HCOOH��NaOHǡ����ȫ��Ӧ
C��NaOH����
CO��H2��Ӧ���Ƶü״���CO+2H2
 CH3OH
CH3OHͼ1��ʾ�÷�Ӧ���й����е������仯��

ͼ2��ʾ100��C�������Ϊ2L�ĺ��������м���4molH2��һ������CO��CO��CH3OH��g����Ũ����ʱ��仯�����
��1����֪CO��ȼ����Ϊ283kJ/mol��H2��ȼ����Ϊ285.8kJ/mol�����ͼ1д��Һ̬CH3OHȼ���ȵ��Ȼ�ѧ����ʽ ��
��2��������ͼ2���㣺���¶��£���ӦCO��g��+2H��g��
 CH3OH��g����ƽ�ⳣ��Ϊ ��10min�������ڵ�ѹǿ��Ϊԭ���� �����ı����������������COת���ʵ��� ��
CH3OH��g����ƽ�ⳣ��Ϊ ��10min�������ڵ�ѹǿ��Ϊԭ���� �����ı����������������COת���ʵ��� ��A�������¶� B���Ӵ���
C�����������ʹ��ϵѹǿ���� D���ٳ���1molCO��2molH2
E�����º��ݸ�Ϊ���º�ѹ
��3����֪��CH3OH������һ��������ת��ΪHCOOH��HCOOH��CH3COOH�������ơ�25��C��0.1mol/LHCOOH��Һ��pH>1�������£���0.1mol/L��HCOOH��Һ�еμ�NaOH��Һ������Һ������Ũ�ȹ�ϵ���㣺c��HCOO-��<c��Na+��ʱ����Ӧ���������Ϊ ��������ĸ��
A��NaOH���㣬HCOOHʣ��
B��HCOOH��NaOHǡ����ȫ��Ӧ
C��NaOH����
��1��CH3OH(l) + 3/2O2(g) = CO2(g) + 2H2O (l) ��H= -725.6kJ/mol
��2��12 0.5 A��D��E ��3��A��B��C
��2��12 0.5 A��D��E ��3��A��B��C
��1������CO��H2��ȼ���Ȳ����ͼ1�ɵã�Һ̬CH3OHȼ���ȵ��Ȼ�ѧ����ʽΪ��
CH3OH(l) + 3/2O2(g) = CO2(g) + 2H2O (l) ��H= -725.6kJ/mol��
��2����ͼ2���㣺���¶��£���ӦCO��g��+2H��g�� CH3OH��g����ƽ�ⳣ��Ϊ12��10min�������ڵ�ѹǿ��Ϊԭ����0.5���������COת���ʵ��Ǽ�ʹƽ��������Ӧ�����ƶ����У�A��D��E���֣�
CH3OH��g����ƽ�ⳣ��Ϊ12��10min�������ڵ�ѹǿ��Ϊԭ����0.5���������COת���ʵ��Ǽ�ʹƽ��������Ӧ�����ƶ����У�A��D��E���֣�
��3�������£���0.1mol/L��HCOOH��Һ�еμ�NaOH��Һ������Һ������Ũ�ȹ�ϵ���㣺c��HCOO-��<c��Na+��ʱ����Ӧ���������Ϊ��A��B��C���������
CH3OH(l) + 3/2O2(g) = CO2(g) + 2H2O (l) ��H= -725.6kJ/mol��
��2����ͼ2���㣺���¶��£���ӦCO��g��+2H��g��
 CH3OH��g����ƽ�ⳣ��Ϊ12��10min�������ڵ�ѹǿ��Ϊԭ����0.5���������COת���ʵ��Ǽ�ʹƽ��������Ӧ�����ƶ����У�A��D��E���֣�
CH3OH��g����ƽ�ⳣ��Ϊ12��10min�������ڵ�ѹǿ��Ϊԭ����0.5���������COת���ʵ��Ǽ�ʹƽ��������Ӧ�����ƶ����У�A��D��E���֣���3�������£���0.1mol/L��HCOOH��Һ�еμ�NaOH��Һ������Һ������Ũ�ȹ�ϵ���㣺c��HCOO-��<c��Na+��ʱ����Ӧ���������Ϊ��A��B��C���������

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ
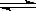 2NH3��g������H��0���ﵽ��ƽ�⡣�ڽ��ı�ijһ�����ﵽ��ƽ�⣬�ı����һ������
2NH3��g������H��0���ﵽ��ƽ�⡣�ڽ��ı�ijһ�����ﵽ��ƽ�⣬�ı����һ������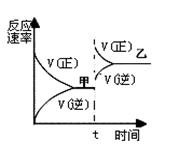
 Fe(SCN)3+3KCl�м���KCl���壬ƽ�⽫���淴Ӧ�����ƶ�����Һ��ɫ����dz
Fe(SCN)3+3KCl�м���KCl���壬ƽ�⽫���淴Ӧ�����ƶ�����Һ��ɫ����dz 2C(g)����H<0����ƽ�������������¶Ƚ��ͣ����������в���ȷ����
2C(g)����H<0����ƽ�������������¶Ƚ��ͣ����������в���ȷ����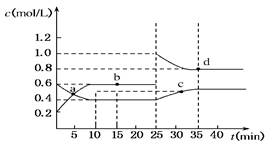

 ?CO2(g)��NO(g)��Ӧ�����������仯ʾ��ͼ��һ�������£��ڹ̶��ݻ����ܱ������и÷�Ӧ�ﵽƽ��״̬�����ı�����һ������X��Y��X�ı仯��ϵ������ͼ�������й�˵����ȷ����(����)
?CO2(g)��NO(g)��Ӧ�����������仯ʾ��ͼ��һ�������£��ڹ̶��ݻ����ܱ������и÷�Ӧ�ﵽƽ��״̬�����ı�����һ������X��Y��X�ı仯��ϵ������ͼ�������й�˵����ȷ����(����)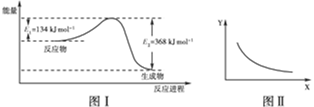
 CO(g) + H2(g)
CO(g) + H2(g) 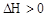 ���ı�������������1���������¶ȣ�����Ӧ���� ���淴Ӧ���� ����������С�䣩��ƽ�� �ƶ���
���ı�������������1���������¶ȣ�����Ӧ���� ���淴Ӧ���� ����������С�䣩��ƽ�� �ƶ���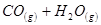

 ��H
��H
 ����ʹ
����ʹ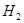 ��ƽ��Ũ������һ������������������ʱ�����д�ʩ���Բ��õ���
��ƽ��Ũ������һ������������������ʱ�����д�ʩ���Բ��õ���
 ��
��
 ��
��