��Ŀ����
ͼ����NO2(g)��CO(g)?
 ?CO2(g)��NO(g)��Ӧ�����������仯ʾ��ͼ��һ�������£��ڹ̶��ݻ����ܱ������и÷�Ӧ�ﵽƽ��״̬�����ı�����һ������X��Y��X�ı仯��ϵ������ͼ�������й�˵����ȷ����(����)
?CO2(g)��NO(g)��Ӧ�����������仯ʾ��ͼ��һ�������£��ڹ̶��ݻ����ܱ������и÷�Ӧ�ﵽƽ��״̬�����ı�����һ������X��Y��X�ı仯��ϵ������ͼ�������й�˵����ȷ����(����)

 ?CO2(g)��NO(g)��Ӧ�����������仯ʾ��ͼ��һ�������£��ڹ̶��ݻ����ܱ������и÷�Ӧ�ﵽƽ��״̬�����ı�����һ������X��Y��X�ı仯��ϵ������ͼ�������й�˵����ȷ����(����)
?CO2(g)��NO(g)��Ӧ�����������仯ʾ��ͼ��һ�������£��ڹ̶��ݻ����ܱ������и÷�Ӧ�ﵽƽ��״̬�����ı�����һ������X��Y��X�ı仯��ϵ������ͼ�������й�˵����ȷ����(����)
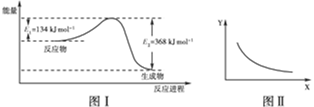
| A���÷�Ӧ���ʱ䦤H����234 kJ��mol��1 |
| B����X��ʾ��ϵ��ѹǿ����Y��ʾ�Ŀ�����NO2��ת���� |
| C����X��ʾ�¶���Y��ʾ�Ŀ�����CO2���ʵ���Ũ�� |
| D������CO����ʼŨ�ȣ�ƽ��������Ӧ�����ƶ�����Ӧ������ |
C
A���÷�Ӧ���ʱ䦤H��-234 kJ��mol��1,���Ǹ����ȷ�Ӧ��
B����X��ʾ��ϵ��ѹǿ����ѹƽ�ⲻ�ƶ���
D������CO����ʼŨ�ȣ�ƽ��������Ӧ�����ƶ�����Ӧ�ų�����������
B����X��ʾ��ϵ��ѹǿ����ѹƽ�ⲻ�ƶ���
D������CO����ʼŨ�ȣ�ƽ��������Ӧ�����ƶ�����Ӧ�ų�����������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 2C(g) + D(g) ��H>0�������淴Ӧ���ʵ�Ӱ�죬���߽����ʾ����ƽ��ʱ���¶Ȼ�ѹǿ��������ȷ����
2C(g) + D(g) ��H>0�������淴Ӧ���ʵ�Ӱ�죬���߽����ʾ����ƽ��ʱ���¶Ȼ�ѹǿ��������ȷ����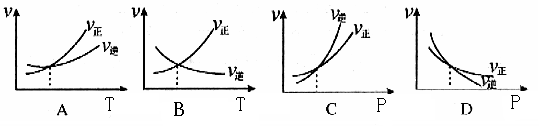
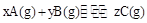
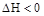 ���ﵽ��ѧƽ�����A�����Ũ��Ϊ
���ﵽ��ѧƽ�����A�����Ũ��Ϊ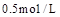 ���������½��ܱ��������ݻ�����һ�����ٴδﵽƽ��ʱ�����A�����Ũ��Ϊ
���������½��ܱ��������ݻ�����һ�����ٴδﵽƽ��ʱ�����A�����Ũ��Ϊ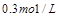 ��������������ȷ���ǣ� ��
��������������ȷ���ǣ� ��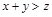
 CH3OH
CH3OH
 CH3OH��g����ƽ�ⳣ��Ϊ ��10min�������ڵ�ѹǿ��Ϊԭ���� �����ı����������������COת���ʵ��� ��
CH3OH��g����ƽ�ⳣ��Ϊ ��10min�������ڵ�ѹǿ��Ϊԭ���� �����ı����������������COת���ʵ��� �� 2C(g)�ﵽƽ��ı�־�ǣ� ��
2C(g)�ﵽƽ��ı�־�ǣ� �� 2NH3(g) ��H =��92.4 kJ��mol-1�����¶ȡ��ݻ���ͬ��2���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������£�
2NH3(g) ��H =��92.4 kJ��mol-1�����¶ȡ��ݻ���ͬ��2���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������£� mZ(g)����H����a kJmol��1(a>0)�����мס������ݻ�����ҹ̶����ܱ��������ڱ��ָ��¶Ⱥ㶨�������£����ܱ���������ͨ��2 mol X��1 mol Y���ﵽƽ��״̬ʱ���ų�����b kJ�����ܱ���������ͨ��1 mol X��0.5 mol Y���ﵽƽ��ʱ���ų�����c kJ����b>2c����a��b��m��ֵ���ϵ��ȷ����
mZ(g)����H����a kJmol��1(a>0)�����мס������ݻ�����ҹ̶����ܱ��������ڱ��ָ��¶Ⱥ㶨�������£����ܱ���������ͨ��2 mol X��1 mol Y���ﵽƽ��״̬ʱ���ų�����b kJ�����ܱ���������ͨ��1 mol X��0.5 mol Y���ﵽƽ��ʱ���ų�����c kJ����b>2c����a��b��m��ֵ���ϵ��ȷ����