��Ŀ����
����Ŀ����ͼ�У�AΪһ����ѧ��ѧ�г����ĵ��ʣ�B��C��D��E�Ǻ���AԪ�صij�����������ǵ���ɫ��Ӧ��Ϊ��ɫ��
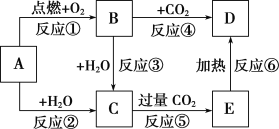
(1)����6����Ӧ������������ԭ��Ӧ����________(��д���)��
(2)B��������������Ŀ��Ϊ________��
(3)д��A��C��Ӧ�Ļ�ѧ����ʽ__________________________________��
(4)д��B��C��Ӧ�����ӷ���ʽ��________________________________��
(5)д��C��E��Ӧ�����ӷ���ʽ��________________________________��
(6)����5.00 g D��E�Ĺ������ʹE��ȫ�ֽ⣬�������������������0.62 g����ԭ�������D����������Ϊ_________��
���𰸡��٢ڢۢ� 1��2 2Na+2H2O��2NaOH+H2�� 2Na2O2��2H2O��4Na����4OH����O2�� OH�� + CO2��HCO3- 66.4%
��������
A��B��C��D��E����ɫ��ӦΪ��ɫ��˵����������Ԫ�أ�AΪ���ʣ���AΪNa������ת����ϵ���Ƴ�BΪNa2O2��CΪNaOH��DΪNa2CO3��EΪNaHCO3���ݴ˷�����
��1����Ӧ����Na��O2��Ӧ��2Na��O2 ![]() Na2O2������������ԭ��Ӧ����Ӧ��Ϊ2Na��2H2O=2NaOH��H2��������������ԭ��Ӧ����Ӧ��2Na2O2��2H2O=4NaOH��O2��������������ԭ��Ӧ����Ӧ��Ϊ2Na2O2��2CO2=2Na2CO3��O2��������������ԭ��Ӧ����Ӧ��NaOH��CO2=NaHCO3��������������ԭ��Ӧ����Ӧ����2NaHCO3
Na2O2������������ԭ��Ӧ����Ӧ��Ϊ2Na��2H2O=2NaOH��H2��������������ԭ��Ӧ����Ӧ��2Na2O2��2H2O=4NaOH��O2��������������ԭ��Ӧ����Ӧ��Ϊ2Na2O2��2CO2=2Na2CO3��O2��������������ԭ��Ӧ����Ӧ��NaOH��CO2=NaHCO3��������������ԭ��Ӧ����Ӧ����2NaHCO3 ![]() Na2CO3��CO2����H2O��������������ԭ��Ӧ��
Na2CO3��CO2����H2O��������������ԭ��Ӧ��
��2����������������BΪ�������ƣ������ʽΪ![]() ���������Ӹ�����Ϊ1��2��
���������Ӹ�����Ϊ1��2��
��3������(1)�ķ�������Ӧ�ڵĻ�ѧ����ʽΪ2Na��2H2O=2NaOH��H2����
��4������(1)�ķ�������Ӧ�۵����ӷ���ʽΪ2Na2O2��2H2O=4Na����4OH����O2����
��5����Ӧ�ݵ����ӷ���ʽΪOH����CO2=HCO3����
��6����������������DΪNa2CO3��EΪNaHCO3�����ȸû�������2NaHCO3 ![]() Na2CO3��CO2����H2O�����У�
Na2CO3��CO2����H2O������
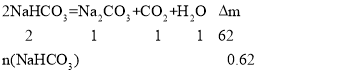
���n(NaHCO3)=0.02mol����Na2CO3��������Ϊ![]() ��100%=66.4%��
��100%=66.4%��

����Ŀ��ijС�鱨�ú�ϡ�����KMnO4����Һ��H2C2O4��Һ�����ᣩ�ķ�Ӧ���˷�ӦΪ���ȷ�Ӧ����̽���������Ի�ѧ��Ӧ���ʵ�Ӱ�죢�������������ķ�����¼ʵ������������Һ�������仯������ѡ�Լ�������:0.2mol L-1H2C2O4��Һ��0.010mol��L-1KMnO4��Һ�����ԣ�������ˮ���Թܡ���Ͳ�����������ˮԡ��
��Ŀ | V(0.2mol L-1H2C2O4��Һ��/mL | V(����ˮ��/mL | V(0.010mol��L-1KMnO4��Һ��/mL | M(MnSO4���壩/g | T/�� | �� |
�� | 2.0 | 0 | 4.0 | 0 | 50 | |
�� | 2.0 | 0 | 4.0 | 0 | 25 | |
�� | 1.0 | a | 4.0 | 0 | 25 | |
�� | 2.0 | 0 | 4.0 | 0.1 | 25 |
�ش���������
��1���������Ӧԭ���Ļ�ѧ��Ӧ����ʽ____________________________________
��2������ʵ��٢���̽��___________�Ի�ѧ��Ӧ���ʵ�Ӱ�죻������ʵ��ڢ���̽��Ũ�ȵĶԻ�ѧ��Ӧ���ʵ�Ӱ�죬��aΪ______������ʵ����Ҫ���������������������������Ӧ��д_______������ʵ��ڢ���̽��__________�Ի�ѧ��Ӧ���ʵ�Ӱ��
��3����֪����Ϊ��Ԫ���ᣬ����뷽��ʽΪ___________________________