��Ŀ����
����Ŀ����������(ClO2) ��Ϊһ�ָ�Чǿ���������ѱ����Ϲ�����������֯(WHO)��ΪAI����ȫ��������ij�о�С�������ͼ��ʾװ���Ʊ�ClO2��NaClO2����֪: ClO2�۵�һ59�����е�11����������ClO2Ϊ����ɫ���ٻ�ɫ���壬ClO2Ũ�ȹ��������ֽ⣬�����ᱬը��NaClO2 ����60��ʱ�ֽ�����NaClO3��NaCl��
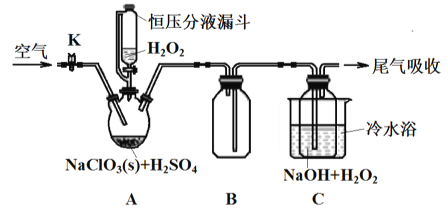
��1��װ�����Ӻú���ҩƷǰ����еIJ�����____________________________________��
��2��ʹ�ú�ѹ��Һ©����Ŀ����_________________������B ��������_________________��
��3��A �з�ӦΪ2NaClO3+H2O2+H2SO4=2C1O2��+O2��+Na2SO4+2H2O��
C �з�Ӧ�Ļ�ѧ����ʽ��_______________�� ��ˮԡ��Ŀ����_________________________��
��4�� ʵ���г���ͨ�������ϡ�����ɵ�ClO2�������ŵ�C����������������̫��������ɵĺ����_______________________________________��
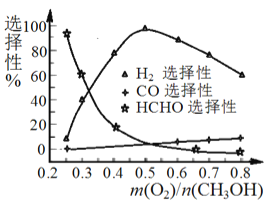
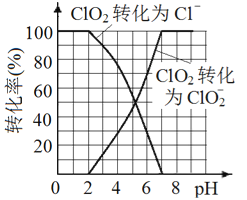
��5����֪: ClO2��I-��ԭΪClO2-��Cl-��ת��������ҺpH �Ĺ�ϵ��ͼ��ʾ����pH��2.0 ʱ��ClO2-Ҳ�ܱ�I- ��ȫ��ԭΪCl-����Ӧ���ɵ�I2��Na2S2O3 ��Ӧ�ķ���ʽ: 2Na2S2O3+ I2= Na2S4O6 + 2NaI��
��ClO2����������ˮ�к���ClO2��ClO2-���ⶨ����ˮ��ClO2��ClO2-�ĺ���������������:
��������ֹ��ȷ����ˮ����ClO2�ĺ���Ϊamol/L��
���õζ�������ClO2-�ĺ������������Ӧ��ʵ�鲽��:
����1: ȷ��ȡVmL ����ˮ��������ƿ�С�
����2: ����ˮ����pH________��
����3: ����������KI ���壬��ַ�Ӧ��
����4: ��������������Һ����cmol/L Na2S2O3 ��Һ�ζ����յ㣬����Na2S2O3 ��ҺV1mL��
���������������ݣ���ø�����ˮ��ClO2-��Ũ��Ϊ____mol/L( �ú���ĸ�Ĵ���ʽ��ʾ)��
���𰸡� ����װ�������� ƽ��ѹǿ������H2O2�ļ��� ��ֹ����(��ȫƿ) 2C1O2+H2O2+2NaOH=2NaClO2+O2��+2H2O ����(���ֹ)H2O2��ClO2��NaClO2�ķֽ� ClO2��Ũ�ȸ��ֽ� <2.0 (cV1-5aV)/4V
����������1�����������ɻ�����ʵ�飬װ�����Ӻú���ҩƷǰ����еIJ����Ǽ���װ�������ԣ���2��ʹ�ú�ѹ��Һ©����Ŀ����ƽ��ѹǿ������H2O2�ļ���������B�������Ƿ�ֹ��������ȫƿ��������3��A�з�ӦΪ2NaClO3+H2O2+H2SO4=2C1O2��+O2��+Na2SO4+2H2O��C��C1O2��˫��ˮ�ڼ��������·�Ӧ����NaClO2����������Ӧ�Ļ�ѧ����ʽ��2C1O2+H2O2+2NaOH=2NaClO2+O2��+2H2O����ˮԡ��Ŀ���Ǽ���(���ֹ)H2O2��ClO2��NaClO2�ķֽ�����4��ʵ���г���ͨ�������ϡ�����ɵ�ClO2�������ŵ�C����������������̫��������ɵĺ����ClO2��Ũ�ȸ��ֽ�����5������ͼ����Ϣ��֪������ˮ����pH <2.0ʱ��ClO2-Ϊ��������ȷ�ⶨClO2-�ĺ��������ݷ�Ӧ2Na2S2O3+I2=Na2S4O6+2NaI��ClO2-+4I-=2I2+Cl-��2ClO2+10I-=5I2+2Cl-�ɵù�ϵClO2-~~~2I2~~~~4Na2S2O3��������ˮ��ClO2-��Ũ��Ϊ![]() ��
��