��Ŀ����
��Ȼ��Ŀ����ʯ�ij���ͱ仯�ܵ�����������Ӱ�졣�ؿ���ÿ����1km��ѹǿ����Լ25000��30000 kPa���ڵؿ���SiO2��HF��������ƽ�⣺SiO2 (s) + 4HF(g)  SiF4 (g) + 2H2O(g)�����÷�Ӧ�ں��º��ݵ��ܱ������з����������жϷ�Ӧ�Ѿ��ﵽ��ѧƽ����ǣ� ��
SiF4 (g) + 2H2O(g)�����÷�Ӧ�ں��º��ݵ��ܱ������з����������жϷ�Ӧ�Ѿ��ﵽ��ѧƽ����ǣ� ��
 SiF4 (g) + 2H2O(g)�����÷�Ӧ�ں��º��ݵ��ܱ������з����������жϷ�Ӧ�Ѿ��ﵽ��ѧƽ����ǣ� ��
SiF4 (g) + 2H2O(g)�����÷�Ӧ�ں��º��ݵ��ܱ������з����������жϷ�Ӧ�Ѿ��ﵽ��ѧƽ����ǣ� ��| A��v��(HF)= 2v��(H2O) | B�����������ܶȱ��ֲ��� |
| C��SiO2���������ֲ��� | D����Ӧ�ﲻ��ת��Ϊ������ |
D
��һ�������£������淴Ӧ������Ӧ���ʺ��淴Ӧ�������ʱ������Ϊ0������Ӧ��ϵ�и������ʵ�Ũ�Ȼ������ٷ����仯��״̬����Ϊ��ѧƽ��״̬������C����˵����D�Ǵ���ģ�A�з�Ӧ���ʵķ����෴������������֮������Ӧ�Ļ�ѧ������֮�ȣ�����˵�����ܶ��ǻ�����������������ݻ��ı�ֵ���ڷ�Ӧ�������������ݻ����DZ仯�ģ�����B����˵������ѡD��

��ϰ��ϵ�д�
�����Ŀ
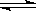 p C��ij�¶��´ﵽƽ�⡣
p C��ij�¶��´ﵽƽ�⡣ H2��g��+I2��g����H2���ʵ�����ʱ��ı仯��ͼ��ʾ��
H2��g��+I2��g����H2���ʵ�����ʱ��ı仯��ͼ��ʾ��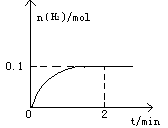
 ?pC(g)��qD(g)(����ͼ)��ʾ����ת������ѹǿ���¶ȵĹ�ϵ������ͼ�����߿��Եó��Ľ�����(����)
?pC(g)��qD(g)(����ͼ)��ʾ����ת������ѹǿ���¶ȵĹ�ϵ������ͼ�����߿��Եó��Ľ�����(����)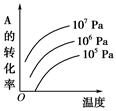
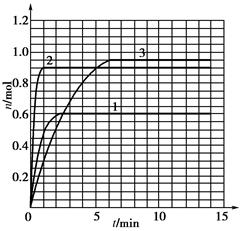
 2NH3��g������H= ��92��4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯��ͼ��
2NH3��g������H= ��92��4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯��ͼ��
 2NH3��g�� +
2NH3��g�� + 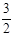 O2��g�� ����H =" a" kJ��mol��1
O2��g�� ����H =" a" kJ��mol��1 N2O4��H��0��������˵����ȷ����
N2O4��H��0��������˵����ȷ����
 2Z + W������ӦΪ���ȷ�Ӧ��(�����ʾ�Ϊ����)���ﵽƽ��ʱ��VA="1.2a" L���Իش� (A�ڵĻ������ƶ�)
2Z + W������ӦΪ���ȷ�Ӧ��(�����ʾ�Ϊ����)���ﵽƽ��ʱ��VA="1.2a" L���Իش� (A�ڵĻ������ƶ�) 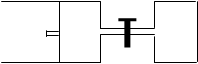
 5Ca2��(aq)��3PO(aq)��OH��(aq)��Ksp��2.5��10��59����ʳ��ϸ����ø������ʳ������л��ᣬ��ʱ���ݾͻ��ܵ���ʴ����ԭ������������������������
5Ca2��(aq)��3PO(aq)��OH��(aq)��Ksp��2.5��10��59����ʳ��ϸ����ø������ʳ������л��ᣬ��ʱ���ݾͻ��ܵ���ʴ����ԭ������������������������