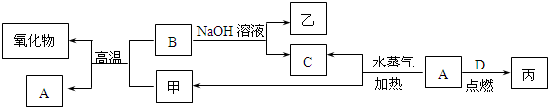
��Ŀ����
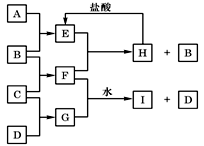 A��B��C��D�ǰ�ԭ��������С�������еĵڶ���������Ԫ�صĵ��ʡ�B��E��Ϊ��ɿ����ijɷ֡�F����ɫ��Ӧ�ʻ�ɫ����G�У��ǽ���Ԫ�������Ԫ�ص�ԭ�Ӹ�����Ϊ1��2����һ�������£�������֮����ת����ϵ���ң�ͼ�в��ֲ���δ�г�����
A��B��C��D�ǰ�ԭ��������С�������еĵڶ���������Ԫ�صĵ��ʡ�B��E��Ϊ��ɿ����ijɷ֡�F����ɫ��Ӧ�ʻ�ɫ����G�У��ǽ���Ԫ�������Ԫ�ص�ԭ�Ӹ�����Ϊ1��2����һ�������£�������֮����ת����ϵ���ң�ͼ�в��ֲ���δ�г�����
![]() ����д���пհף�
����д���пհף�
![]() ��1��A�� ��C�� ��
��1��A�� ��C�� ��
![]() ��2��H�����ᷴӦ����E�Ļ�ѧ����ʽ�� ��
��2��H�����ᷴӦ����E�Ļ�ѧ����ʽ�� ��
![]() ��3��E��F��Ӧ�Ļ�ѧ����ʽ�� ��
��3��E��F��Ӧ�Ļ�ѧ����ʽ�� ��
![]() ��4��F��G��ˮ��Һ��Ӧ����I��D�����ӷ���ʽ�� ��
��4��F��G��ˮ��Һ��Ӧ����I��D�����ӷ���ʽ�� ��
��1��A��̼(��C) C����(��Na)����2��Na2CO3+2HCl![]() 2NaCl+H2O+CO2��
2NaCl+H2O+CO2��
![]() ��3��2CO2+2Na2O2
��3��2CO2+2Na2O2![]() 2Na2CO3+O2����4��Na2O2+S2-+2H2O
2Na2CO3+O2����4��Na2O2+S2-+2H2O![]() 4OH-+S��+2Na+
4OH-+S��+2Na+
����:
F����ɫ��Ӧ�ʻ�ɫ��˵��F���ƵĻ������F�ǵ���B��C���϶��ɣ�B��E��Ϊ��ɿ����ijɷ֣���CΪ�����ƣ�G�ǵ���C��D���϶��ɣ�ԭ�Ӹ�����ΪD�� C��1��2����ԭ������D��C����DΪ��BΪ��ɿ����ijɷ֣��������뵽����O2����BΪO2��EҲΪ��ɿ����ijɷ֣�Eֻ����CO2��A��Ϊ̼��F��Na2O2����Na2O����ϵ��F+E��H+B����F��Na2O2��H��Na2CO3��