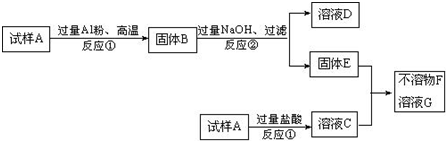
��Ŀ����
ȡ����Fe2O3��ĩ������ɫ�������������ᣬ��������Ӧ�Ļ�ѧ����ʽΪ______����Ӧ��õ���FeCl3��Һ�ʻ�ɫ���ô���Һ��������ʵ�飺
��1��ȡ������Һ�����Թ��У�����NaOH��Һ�����Թ۲쵽�к��ɫ�������ɣ���Ӧ�Ļ�ѧ����ʽΪ______���˷�Ӧ�����ӷ���ʽΪ______��
��2����С�ձ��м���25mL������ˮ�����������ں����ˮ�м���2mLFeCl3��Һ�������������Һ��______ɫ�������Ƶ�Fe��OH��3���壮
��3����ȡһС�ձ�Ҳ����25mL����ˮ�����ձ���Ҳ����2mLFeCl3��Һ�����Ⱥ����ձ�����żף���ʢ��Fe��OH��3������ձ�������ң�һ����ð������ֱ��ú�ɫ����������ձ��е�Һ�壬�������______�����ֱ����ƽ�С����ĽǶȣ����Կ���______����ס����ҡ����ձ��л���������ЧӦ��
��1��ȡ������Һ�����Թ��У�����NaOH��Һ�����Թ۲쵽�к��ɫ�������ɣ���Ӧ�Ļ�ѧ����ʽΪ______���˷�Ӧ�����ӷ���ʽΪ______��
��2����С�ձ��м���25mL������ˮ�����������ں����ˮ�м���2mLFeCl3��Һ�������������Һ��______ɫ�������Ƶ�Fe��OH��3���壮
��3����ȡһС�ձ�Ҳ����25mL����ˮ�����ձ���Ҳ����2mLFeCl3��Һ�����Ⱥ����ձ�����żף���ʢ��Fe��OH��3������ձ�������ң�һ����ð������ֱ��ú�ɫ����������ձ��е�Һ�壬�������______�����ֱ����ƽ�С����ĽǶȣ����Կ���______����ס����ҡ����ձ��л���������ЧӦ��
�������������ᷴӦ�����κ�ˮ��Fe2O3�����ᷴӦ����FeC13��H2O����Ӧ�Ļ�ѧ����ʽΪ��Fe2O3+6HC1=2FeC13+3H2O��
�ʴ�Ϊ��Fe2O3+6HC1=2FeC13+3H2O��
��1���κͼӦ�����¼�����Σ�FeC13��Һ��NaOH��Һ��Ӧ�����ɺ��ɫ����Fe��OH��3��H2O��
��Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ�ֱ�ΪFeC13+3NaOH=Fe��OH��3��+3NaC1��Fe3++3OH-=Fe��OH��3����
�ʴ�Ϊ��FeC13+3NaOH=Fe��OH��3��+3NaC1��Fe3++3OH-=Fe��OH��3����
��2��Fe��OH��3������ɫΪ���ɫ���ʴ�Ϊ����֣�
��3���������ЧӦʵ�飬Ϊ�˱��ڹ۲��·Ӧ������ߴ�ֱ�Ƕȹ۲죬����ΪFeC13��Һ������ΪFe��OH��3���壬�����ܲ��������ЧӦ��
�ʴ�Ϊ����ֱ���ң�
�ʴ�Ϊ��Fe2O3+6HC1=2FeC13+3H2O��
��1���κͼӦ�����¼�����Σ�FeC13��Һ��NaOH��Һ��Ӧ�����ɺ��ɫ����Fe��OH��3��H2O��
��Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ�ֱ�ΪFeC13+3NaOH=Fe��OH��3��+3NaC1��Fe3++3OH-=Fe��OH��3����
�ʴ�Ϊ��FeC13+3NaOH=Fe��OH��3��+3NaC1��Fe3++3OH-=Fe��OH��3����
��2��Fe��OH��3������ɫΪ���ɫ���ʴ�Ϊ����֣�
��3���������ЧӦʵ�飬Ϊ�˱��ڹ۲��·Ӧ������ߴ�ֱ�Ƕȹ۲죬����ΪFeC13��Һ������ΪFe��OH��3���壬�����ܲ��������ЧӦ��
�ʴ�Ϊ����ֱ���ң�

��ϰ��ϵ�д�
 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
�����Ŀ