ƒøƒ⁄»ð
°æƒø°øÌ⁄(Te)Œ™µ⁄ŒÂ÷Ð∆⁄‘™Àÿ£¨”ΗıÕ¨÷˜◊£¨∆‰µ•÷ ∫ÕªØ∫œŒÔ‘⁄ªØπ§…˙≤˙µ»∑Ω√Êæþ”–π„∑∫”¶”√°£
£®1£©ª≠≥ˆÌ⁄µƒ‘≠◊”Ω·ππ æ“‚Õº___________________°£
£®2£©“—÷™TeO2Œ¢»Ð”⁄ÀÆ£¨“◊»Ð”⁄Ωœ≈®µƒ«øÀ·∫Õ«øºÓ°£–¥≥ˆTeO2»Ð”⁄≈®«‚—ıªØƒ∆»Ð“∫µƒ¿Î◊”∑Ω≥à Ω_________________________°£
£®3£©π§“µ…œ”√Õ≠—Ùº´ƒý(÷˜“™≥…∑÷Œ™Cu2Te£¨ªπ∫¨”–…Ÿ¡øµƒAg°¢Au)Œ™‘≠¡œ÷∆±∏µ•÷ Ì⁄µƒπ§“’¡˜≥ûÁœ¬£∫

¢Ÿ°∞º”—πΩ˛≥ˆ°±π˝≥Ã÷–±ª—ıªØµƒ‘™ÀÿŒ™_____________(ÃÓ‘™Àÿ∑˚∫≈)£¨1molCu2Te±ª°∞Ω˛≥ˆ°± ±—ıªØº¡µ√µΩµƒµÁ◊” ˝Œ™___________________°£
¢⁄°∞À·Ω˛°± ±£¨Œ¬∂»π˝∏þª· πÌ⁄µƒΩ˛≥ˆ¬ ΩµµÕ£¨‘≠“ÚŒ™____________________°£
¢€°∞ªπ‘≠°±π˝≥õƒªØ—ß∑Ω≥Ã ΩŒ™____________________°£
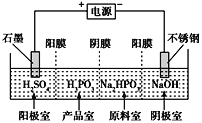
¢Ð𧓵…œªπø…“‘Ω´Õ≠—Ùº´ƒýÏ—…’°¢ºÓΩ˛∫Ûµ√µΩNa2TeO3»Ð“∫£¨»ª∫Û‘ŸÕ®π˝µÁΩ‚µƒ∑Ω∑®µ√µΩµ•÷ Ì⁄£¨“ıº´µƒµÁº´∑¥”¶ ΩŒ™______________________ °£
£®4£©25°Ê ±£¨œÚ1mol°§L-1µƒNa2TeO3»Ð“∫÷–µŒº”—ŒÀ·£¨µ±»Ð“∫pH‘ºŒ™6 ±£¨¥À ±»Ð“∫÷–c(HTeO3-)£∫c(TeO32-)=_____________°£(H2TeO3µƒKa1=1.0°¡10-3£¨Ka2=2.0°¡10-8)
°æ¥∞∏°ø  TeO2+2OH-=TeO32-+H2O Cu°¢Te 8NA(ªÚ4.816°¡1024) Œ¬∂»…˝∏þ£¨—ŒÀ·ª”∑¢£¨∑¥”¶ŒÔ≈®∂»ΩµµÕ£¨µº÷¬Ω˛≥ˆ¬ ΩµµÕ TeCl4+2SO2+4H2O=Te°˝+4HCl+2H2SO4 TeO32-+3H2O+4e-=Te+6OH- 50£∫1
TeO2+2OH-=TeO32-+H2O Cu°¢Te 8NA(ªÚ4.816°¡1024) Œ¬∂»…˝∏þ£¨—ŒÀ·ª”∑¢£¨∑¥”¶ŒÔ≈®∂»ΩµµÕ£¨µº÷¬Ω˛≥ˆ¬ ΩµµÕ TeCl4+2SO2+4H2O=Te°˝+4HCl+2H2SO4 TeO32-+3H2O+4e-=Te+6OH- 50£∫1
°æΩ‚Œˆ°ø£®1£©Ì⁄(Te)Œ™µ⁄ŒÂ÷Ð∆⁄‘™Àÿ£¨”ΗıÕ¨÷˜◊£¨∆‰‘≠◊”–Ú ˝Œ™£∫52£¨π ∆‰‘≠◊”µÁ◊”≈≈≤ºŒ™£∫2 8 18 18 6£¨‘≠◊”Ω·ππ æ“‚ÕºŒ™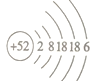 £ª
£ª
£®2£©“—÷™“◊»Ð”⁄Ωœ≈®µƒ«øÀ·∫Õ«øºÓ£¨’‚“ª–‘÷ ”ΗıªØ¬¡µƒ–‘÷ ¿ýÀ∆£¨π TeO2»Ð”⁄≈®«‚—ıªØƒ∆»Ð“∫µƒ¿Î◊”∑Ω≥Ã ΩŒ™£∫TeO2+2OH-=TeO32-+H2O£ª
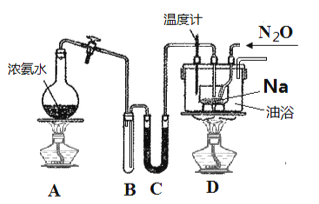
£®3£©¢Ÿ∏˘æð¡˜≥ÃÕº£∫°∞º”—πΩ˛≥ˆ°±µ√µΩ¡ÀCuSO4»Ð“∫∫ÕTeO2£¨Õ≠—Ùº´ƒý÷˜“™≥…∑÷Œ™Cu2Te£¨æ˘Œ™0º€£¨π ∏√π˝≥Ã÷–Cu∫ÕTe±ª—ı∆¯—ıªØ£ª1molCu2Te±ª°∞Ω˛≥ˆ°± ±£¨…˙≥…2mol CuSO4 ◊™“∆4molµÁ◊”∫Õ1mol TeO2 ◊™“∆4molµÁ◊”£¨π 1molCu2Te±ª°∞Ω˛≥ˆ°± ±—ıªØº¡µ√µΩµƒµÁ◊” ˝Œ™8NAªÚ4.816°¡1024£ª
¢⁄°∞À·Ω˛°± ±£¨”√≈®—ŒÀ·Ω´TeO2◊™ªØ≥…TeCl4£¨Œ¬∂»π˝∏þ£¨—ŒÀ·ª”∑¢£¨∑¥”¶ŒÔ≈®∂»ΩµµÕ£¨µº÷¬Ω˛≥ˆ¬ ΩµµÕ£ª
¢€°∞ªπ‘≠°±π˝≥Ô√SO2Ω´TeCl4ªπ‘≠≥…µ•÷ Te£¨π ∏√∑¥”¶µƒ∑Ω≥Ã ΩŒ™£∫TeCl4+2SO2+4H2O=Te°˝+4HCl+2H2SO4£ª
¢Ð𧓵…œªπø…“‘Ω´Õ≠—Ùº´ƒýÏ—…’°¢ºÓΩ˛∫Ûµ√µΩNa2TeO3»Ð“∫£¨»ª∫Û‘ŸÕ®π˝µÁΩ‚Na2TeO3»Ð“∫µƒ∑Ω∑®µ√µΩµ•÷ Ì⁄£¨“ıº´∑¢…˙ªπ‘≠∑¥”¶£¨”–‘™ÀÿªØ∫œº€“™ΩµµÕ£¨º¥ «Te”…+4º€ΩµµΩ0º€£¨π µÁº´∑¥”¶Œ™£∫TeO32-+3H2O+4e- =Te+6OH-£ª
£®4£©25°Ê ±£¨œÚ1mol°§L-1µƒNa2TeO3»Ð“∫÷–µŒº”—ŒÀ·£¨µ±»Ð“∫pH‘ºŒ™6 ±£¨c£®H+£©=10-6mol/L£¨H2TeO3µƒKa2=![]() =2.0°¡10-8£¨π c(HTeO3-)£∫c(TeO32-)=50£∫1°£
=2.0°¡10-8£¨π c(HTeO3-)£∫c(TeO32-)=50£∫1°£

 –°—ß…˙10∑÷÷”ø⁄À„≤‚ ‘100∑÷œµ¡–¥∞∏
–°—ß…˙10∑÷÷”ø⁄À„≤‚ ‘100∑÷œµ¡–¥∞∏°æƒø°øµÕŒ¬∆» π÷≤ŒÔœ∏∞˚≤˙…˙¥Û¡ø∂‘œ∏∞˚”–∫¶µƒπ˝—ıªØŒÔ£¨»Á÷¨÷ π˝—ıªØŒÔ£®MDA£©°£≥¨—ıªØŒÔ∆ÁªØ√∏£®SOD£©ƒÐπªœ˚≥˝π˝—ıªØŒÔ£¨¥”∂¯‘ˆ«ø÷≤ŒÔµƒøπ¿‰–‘°£—–æø»À‘±Ω¯––¡À°∞ÀÆ—ÓÀ·∂‘ÀƵæ”◊√Áøπ¿‰–‘µƒ”∞œÏ°± µ—È£¨ µ—È≤Ω÷˺∞Ω·π˚»Á±ÌÀ˘ æ°£
◊ȱ | ¥¶¿Ì | ≈ý—¯Œ¬∂» /°Ê |
|
1 | ’Ù¡ÛÀÆΩΩπý | 25 | 7£Æ3 |
2 | ¢Ÿ | ¢⁄ | 9£Æ4 |
3 | 0£Æ5 mmol/LÀÆ—ÓÀ·ΩΩπý | 5 | 10£Æ3 |
4 | 1£Æ0 mmol/LÀÆ—ÓÀ·ΩΩπý | 5 | 11£Æ6 |
5 | 1£Æ5 mmol/LÀÆ—ÓÀ·ΩΩπý | 5 | 13£Æ6 |
6 | 2£Æ0 mmol/LÀÆ—ÓÀ·ΩΩπý | 5 | 8£Æ5 |
7 | 2£Æ5 mmol/LÀÆ—ÓÀ·ΩΩπý | 5 | 7£Æ9 |
8 | 3£Æ0 mmol/LÀÆ—ÓÀ·ΩΩπý | 5 | 6£Æ5 |
£®1£©±Ì÷–¢Ÿ « £¨¢⁄ « £¨∆‰µƒ◊˜”√Œ™ £¨ µ—ȅ˺∆ ±√ø◊È»°50÷ÍÀƵæ”◊√Á£¨∂¯≤ª «1÷Í£¨ƒøµƒ « °£
£®2£©±æ µ—È◊‘±‰¡øŒ™ £¨ £¨–Ë“™øÿ÷∆µƒŒÞπÿ±‰¡ø”– £¨ £®÷¡…Ÿ–¥¡Ω∏ˆ£©°£
£®3£©◊ȱ1∫Õ2∂‘’’ø…µ√µƒΩ·¬€ « °£∂‘±»◊ȱ2°´8ø…µ√µƒΩ·¬€ « °£
£®4£©‘⁄5 °Êµƒª∑æ≥œ¬£¨ŒÔ÷ µƒ¡ø≈®∂»Œ™2£Æ0 mmol/LµƒÀÆ—ÓÀ·∂‘ÀƵæ”◊√Áøπ¿‰–‘µƒ”∞œÏ « £®ÃÓ°∞‘ˆ«ø°±ªÚ°∞ºı»ı°±£©°£
£®5£©«Î∏˘æð5 °ÊÃıº˛œ¬µƒ µ—ÈΩ·π˚ÕÍ≥…ÀÆ—ÓÀ·≈®∂»°™SODªÓ–‘πÿœµµƒ◊¯±Í«˙œþÕº°£
°æƒø°ø“—÷™A(g)+B(g)![]() C(g)+D(g)∑¥”¶µƒ∆Ω∫‚≥£ ˝∫ÕŒ¬∂»µƒπÿœµ»Áœ¬£∫
C(g)+D(g)∑¥”¶µƒ∆Ω∫‚≥£ ˝∫ÕŒ¬∂»µƒπÿœµ»Áœ¬£∫
Œ¬∂»/ °Ê | 700 | 800 | 830 | 1000 | 1200 |
∆Ω∫‚≥£ ˝ | 1.7 | 1.1 | 1.0 | 0.6 | 0.4 |
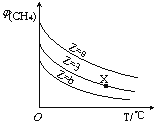
830°Ê ±£¨œÚ“ª∏ˆ2 Lµƒ√б’»ð∆˜÷–≥‰»Î0.2molµƒA∫Õ0.8molµƒB£¨∑¥”¶≥ı º4 sƒ⁄Aµƒ∆Ωæ˘∑¥”¶ÀŸ¬ v(A)=0.005mol/(L°§s)°£œ¬¡–Àµ∑®’˝»∑µƒ «
A£Æ4 s ±c(B)Œ™0.76mol/L
B£Æ830°Ê¥Ô∆Ω∫‚ ±£¨Aµƒ◊™ªØ¬ Œ™80%
C£Æ∑¥”¶¥Ô∆Ω∫‚∫Û£¨…˝∏þŒ¬∂»£¨∆Ω∫‚’˝œÚ“∆∂Ø
D£Æ1200°Ê ±∑¥”¶C(g)+D(g£©![]() A(g)+B(g)µƒ∆Ω∫‚≥£ ˝µƒ÷µŒ™0.4
A(g)+B(g)µƒ∆Ω∫‚≥£ ˝µƒ÷µŒ™0.4
°æƒø°ø25°Ê ±£¨”–πÿŒÔ÷ µƒµÁ¿Î∆Ω∫‚≥£ ˝»Áœ¬£∫
ªØ—ß Ω | CH3COOH | H2CO3 | H2SO3 |
µÁ¿Î∆Ω∫‚≥£ ˝ | K=1.8°¡10£≠5 | K1=4.3°¡10£≠7 K2=5.6°¡10£≠11 | K1=1.5°¡10£≠2 K2=1.02°¡10£≠7 |
(1)«Î–¥≥ˆH2SO3µƒµÁ¿Î∆Ω∫‚≥£ ˝K1µƒ±Ì¥Ô Ω£∫________________°£
(2) ≥£Œ¬œ¬£¨Ω´Ãª˝Œ™10mL pH=2µƒ¥◊À·»Ð“∫”Η«¡ÚÀ·»Ð“∫∑÷±º”’Ù¡ÛÀÆœ° Õ÷¡1000mL£¨œ° Õ∫ۻГ∫µƒpH£¨«∞’þ_____∫Û’þ£®ÃÓ°∞£æ°±°¢°∞£º°±ªÚ°∞=°±£©°£
(3)“ª∂®Ãıº˛œ¬£¨±˘¥◊À·º”ÀÆœ° Õπ˝≥Ã÷–»Ð“∫µºµÁƒÐ¡¶IÀʺ”ÀÆê˝V±‰ªØ«˙œþ»Á”“ÕºÀ˘ 棨‘Úa°¢b°¢c»˝µ„»Ð“∫¥◊À·µƒµÁ¿Î≥Ã∂»”…¥ÛµΩ–°Œ™____________________°£

(4)œ¬¡–¿Î◊”CH3COO£≠°¢CO32£≠°¢HSO3£≠°¢SO32£≠‘⁄»Ð“∫÷–Ω·∫œH£´µƒƒÐ¡¶”…¥ÛµΩ–°µƒπÿœµŒ™___________°£
(5)ê˝œýÕ¨°¢c(H£´)œýÕ¨µƒ¢ŸCH3COOH£ª¢⁄HCl£ª¢€H2SO4 »˝÷÷À·»Ð“∫∑÷±”ÎÕ¨≈®∂»µƒNaOH»Ð“∫ÕÍ»´÷–∫Õ ±£¨œ˚∫ƒNaOH»Ð“∫µƒÃª˝”…¥ÛµΩ–°µƒ≈≈¡–À≥–Ú «_____(ÃÓ–Ú∫≈)°£
(6)“—÷™£¨H£´(aq) + OH£≠(aq) == H2O(l) ¶§H =£≠57.3 kJ/mol°£ µ—È≤‚µ√œ°¥◊À·”Îœ°NaOH»Ð“∫∑¥”¶…˙≥…1 mol H2O ±∑≈≥ˆ57 kJµƒ»»£¨‘Ú¥◊À·»Ð“∫÷–£¨¥◊À·µÁ¿Îµƒ»»ªØ—ß∑Ω≥Ã ΩŒ™________________°£