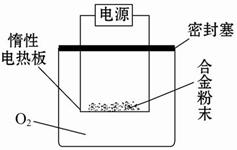
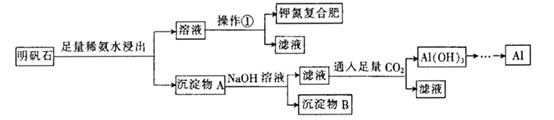
��Ŀ����
���������䡢��ˮ��Ӧ����������ˮ������Ӧ��ʵ��װ����ͼ��ʾ����Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ���������ˮ������Ӧ��ʵ�顣��ش�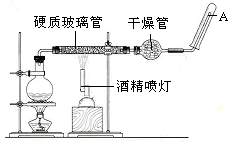
��1��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��2��ʵ��ǰ���������װ�ý��еIJ�����________��ʵ�鿪ʼʱӦ�ȵ�ȼ ����ƾ��ơ��ƾ���ơ�����ʵ�����ʱӦ��Ϩ�� ����ƾ��ơ��ƾ���ơ�����
��3��Բ����ƿ��ʢ��ˮ����װ�����Ⱥ����Ҫ������ ����ƿ��Ӧ���ȷ��� ���������� ���������ʢװ��������________��
��4����Ӳ�ʲ�������ȴ��ȡ�������еĹ�����������ϡ�������ҺB��ȡ������ҺB�μ� ��Һ���� ����ʵ��������˵����ҺB�к���Fe3+��
��1��3Fe+4H2O(g) Fe3O4+4H2����2�����װ�õ������ԣ��ƾ��ƣ��ƾ���ƣ�
Fe3O4+4H2����2�����װ�õ������ԣ��ƾ��ƣ��ƾ���ƣ�
��3��Ϊʵ���ṩˮ���������Ƭ����ֹ���У���ʯ�ң�������Ҳ���֣�����4��KSCN����Һ���ɫ������������Ҳ���֣���
���������������1�������£�����ˮ������Ӧ������������������������ѧ����ʽΪ3Fe+4H2O(g) Fe3O4+4H2����2��ʵ��ǰ���������װ�ý��еIJ����Ǽ��װ�õ������ԣ�ʵ�鿪ʼʱ��Ҫ���ž�װ���еĿ�����Ӧ�ȵ�ȼ�ƾ��ƣ�ʵ�����ʱ��Ӧ�Dz�����ˮ��������ȴ��Ӧ��Ϩ��ƾ���ƣ� ��3��Բ����ƿ��ʢ��ˮ����װ�����Ⱥ����Ҫ������Ϊʵ���ṩˮ��������ƿ��Ӧ���ȷ������Ƭ���������Ƿ����У��������ʢװ�������Ǽ�ʯ�ҡ���4������Fe3+�ļ��飻��Ӳ�ʲ�������ȴ��ȡ�������еĹ�����������ϡ�������ҺB��ȡ������ҺB�μ�KSCN��Һ������Һ���ɫ����˵����ҺB�к���Fe3+��
Fe3O4+4H2����2��ʵ��ǰ���������װ�ý��еIJ����Ǽ��װ�õ������ԣ�ʵ�鿪ʼʱ��Ҫ���ž�װ���еĿ�����Ӧ�ȵ�ȼ�ƾ��ƣ�ʵ�����ʱ��Ӧ�Dz�����ˮ��������ȴ��Ӧ��Ϩ��ƾ���ƣ� ��3��Բ����ƿ��ʢ��ˮ����װ�����Ⱥ����Ҫ������Ϊʵ���ṩˮ��������ƿ��Ӧ���ȷ������Ƭ���������Ƿ����У��������ʢװ�������Ǽ�ʯ�ҡ���4������Fe3+�ļ��飻��Ӳ�ʲ�������ȴ��ȡ�������еĹ�����������ϡ�������ҺB��ȡ������ҺB�μ�KSCN��Һ������Һ���ɫ����˵����ҺB�к���Fe3+��
���㣺���������仯���������ʵ�顣

 �żӾ���ϵ�д�
�żӾ���ϵ�д�ͭ����������������ʹ�õĽ���֮һ���ڻ�ѧ��Ӧ��ͭԪ�ؿɱ���Ϊ0��+1��+2�ۡ�
��1���������ż������м��أ������������Ϊͭ�����������ࣨCuSO4��������Ӧ����ͭ����д���÷�Ӧ�����ӷ���ʽ ��
��2����ԭ��غ͵����У�ͭ�������缫�������й�˵����ȷ����
| A��пͭԭ�����ͭ������ | B���õ�ⷨ����ͭʱ��ͭ������ |
| C���ڶƼ��϶�ͭʱͭ���Դ�������� | D��ͭ������ʱ��һ���ܽ� |
��������⡿̼������ȷֽ�����CaO��CO2����ô��ˮ����ͭ���ȷֽ�Ҳֻ����CuO��SO3��
�����ʵ�顿����ͼװ�ý������顣
|
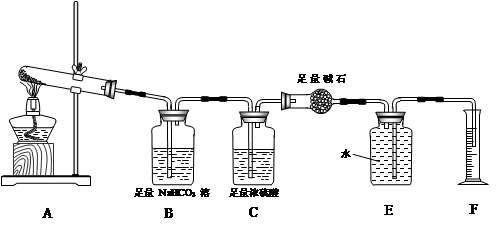 ��ʵ����̡�
��ʵ����̡�a��ȷ��ȡ��ˮ����ͭ2.40g��װ��A�Թ��м��ȣ�ֱ������ȫ����Ϊ��ɫ������֤�ú�ɫ��ĩΪCuO��
b��ʵ���У��۲쵽װ��E�е�ˮ���ֱ�������Ͳ�У�ʵ����������Ͳ��ˮ�����Ϊ112mL��������ɱ�״̬������������������ø����D������������1.32g��
��3��װ��C������ ��
��4������װ��E��F�������Ʋ������ �������ʽ�����ɣ�ʵ�����ɵ�SO3Ϊ mol����5��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ ��
��ʵ����ۡ���ˮ����ͭ���ȷֽⲻ��������CuO��SO3��
����˼�����ۡ�
��6���κ�ʵ�鶼��������ָ����ʵ���п���������������� ����д2�㣩��
ijУ��ѧ��ȤС��Ϊ̽������Ũ���ᷴӦ,�����ͼ1��ͼ2��ʾװ�ý���ʵ�顣

��������ͼ1���������������� ��ͼ2
(1)�Ƚ���ʵ��װ��,ͼ2װ�õ��ŵ���:
���ܸ��õ������ж�����SO2,��ֹ����Ⱦ����;
������
(2)������e��������Ҫ����:һ���ڷ�Ӧ������,�ܲ���Һ����,����Һ�⡱������ֹSO2�����ݳ�����Ⱦ����;��������
(3)��˵����SO2���������ʵ������������
(4)��Ӧһ��ʱ���,�õι���ȡA�Թ��е���Һ��������ˮ��Ϊ����,�����������������ӵijɷ����������ֿ���:
��:ֻ����Fe3+;��:ֻ����Fe2+;��:����Fe3+����Fe2+��
Ϊ��֤��Ŀ�����,ѡ�������Լ�,��д���пո�:
| A��ϡHCl��Һ | B��ϡH2SO4��Һ | C��KSCN��Һ | D��KMnO4��Һ |
��֤��:ȡ����,�ȵμ�������������(���Լ����,��ͬ),��,�ٵμ�������������,������Һ��ɫ�ı仯��ȷ��������Ƿ���ȷ����Ӧ�����ӷ���ʽ��������������������,Fe3++3SCN-
 Fe(SCN)3��
Fe(SCN)3�� ��֤��:����1.ȡ����,�μ�������������(���Լ����),��Һ����ɫ��������ɫ,�������к���Fe3+��
����2.��ȡ�����������μӵ���������������(���Լ����),�۲쵽������Ϊ��������,�������к���Fe2+��
Ϊ�ⶨNa2CO3��NaHCO3����������Na2CO3��������������ȡһ����������Ʒ����ͬѧ����ͼI��ʾװ�ò�������CO2���������ͬѧ����ͼII��ʾװ��ͨ������ܵ����ز�������CO2����������֪����ϡ�����������
��l��ʢ��ϡ�������������Ϊ ��
��2���Լ�XΪ ���Լ�YΪ ��
��3����ͬѧ�ڽ���ʵ��ʱ��Ϊ��С��Ӧע��������У���ѡ����ĸ�� ��
| A������ǰӦʹ����װ����ȴ������ |
| B������Z�ĸ߶�ʹ����װ������Һ����ƽ |
| C������ʱ������Z�ڰ�Һ����͵����� |
| D������ǰӦͨ��һ������N2ʹ���ɵ�CO2ȫ����������װ�� |
FeCl3���ִ���ҵ������Ӧ�ù㷺��ij��ѧ�о���ѧϰС��ģ�ҵ�����Ʊ���ˮFeCl3�����ø���ƷFeCl3��Һ�����ж���H2S��
I�����������ϵ�֪����ˮFeCl3�ڿ������׳��⣬����������������������Ʊ���ˮ FeCl3��ʵ�鷽����װ��ʾ��ͼ�����ȼ��г�װ����ȥ���������������£�
�ټ���װ�õ������ԣ�������������
��ͨ������Cl2���Ͼ�װ���еĿ�����
���þƾ�������м�·���������Ӧ��ɣ�
�ܡ���
����ϵ��ȴ��ֹͣͨ��Cl2�����ø����H2�Ͼ�Cl2�����ռ����ܷ⡣
��ش��������⣺
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ�������������������������������������� ��
��2�����������У�Ϊ��ֹFeCl3��������ȡ�Ĵ�ʩ�У������ţ��������������� ��
��3�����û��װ��C����ƣ��ᵼ��
��4�������ӷ���ʽ��ʾ���߿�E��������װ�ú��Լ������ã� ��
��5����װ��D�еĸ���ƷFeCl3��Һ����H2S���õ���������д����Ӧ�����ӷ���ʽ���������� ��
��Ӧ�������ռ��������ù�����ȫ����ϡ���ᣬС��ͬѧ��������Һ���������ӵijɷ������ֹ۵㣺��ֻ��Fe3+����ֻ��Fe2+������ �� ��������
Ϊ̽����Һ����ɣ�ʵ�����£�
| ʵ�鲽�� | ʵ������ | ʵ����ۼ���Ӧ���ӷ���ʽ |
| ��ȡ����������Һ���Թ��У���������KSCN��Һ�� | _________________�� | ˵��������ڲ�����������ٻ�۳�������Ӧ�����ӷ���ʽ��_____________�� |
| ����ȡ����������Һ���Թ��У������������� KMnO4��Һ�� | ��Һ�Ϻ�ɫ��ȥ | ˵����________________________�� |
�ۺ�����ʵ�������������Һ�к��еĽ����������� ��
 �ⶨʣ�����������ʵ���з�����Ӧ�Ļ�ѧ����ʽ�ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
�ⶨʣ�����������ʵ���з�����Ӧ�Ļ�ѧ����ʽ�ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ� �ⶨ������������ʵ��װ�ã�
�ⶨ������������ʵ��װ�ã�