��Ŀ����
����Ŀ���л�������࣬�㷺�ֲ����в�ș��Ҷ�������ر��ǹ�ʵ�У����л��ϳɡ���ũҵ��������Ҫԭ�ϣ���ش������й����⣺
��1�������Ǻϳ�������������Ҫԭ�ϣ��Ʊ�ԭ�����£�
CH3COOH(l)+ C2H5OH(l)![]() CH3COOHC2H5(l)+H2O(l) ��H=-8.62kJ/mol
CH3COOHC2H5(l)+H2O(l) ��H=-8.62kJ/mol
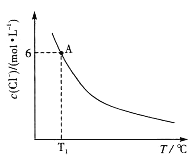
��֪��CH3COOH��C2H5OH��CH3COOC2H5�ķе�����Ϊ118�桢78 ���77�档������������ͬʱ��ij�о�С������˶��ʵ�飬ʵ������ͼ��ʾ��

�ٸ��о�С���ʵ��Ŀ����________________��60���·�Ӧ40min��70���·�Ӧ20min��ȣ�ǰ�ߵ�ƽ����Ӧ����___________���ߣ��С�ڡ��������ڡ����ڡ�����
����ͼ��ʾ����Ӧʱ��Ϊ40min���¶ȳ���80��ʱ���������������½���ԭ�������___________��
�����ô�ԭ���Ƶõ�����������Ʒ�г�����һ���������ᡢ�Ҵ��Լ��������ᣬ�ᴿʱ�����Ʒ�м���__________��Һ���г��ϴ�ӣ�������л��㣬��������ˮ�����Ƹ�����__________��_______�����������ɵõ���Ʒ��
��2���Ҷ����������ᣨ��Ԫ���ᣬ�ṹ��ʽ��HOOC��COOH�������㷺��Ӧ���ڿ�������ҩ��ĺϳɡ�
��ʵ�����о�����������KMnO4��Һ�ζ������䴿�ȡ���֪����������KMnO4��Һ��Ӧ��������ɫ��ζ�����������KMnO4��Һ��ɫ��ȥ��д������������KMnO4��Һ��Ӧ�����ӷ���ʽ_____________________��
�����ϱ�����25��ʱ����ĵ���ƽ�ⳣ��Ka1=6.0��10-2��Ka2=6.4��10-5���ݴ˷���������ʱ������أ�KHC2O4����ˮ�ⷴӦƽ�ⳣ��Kh=________����λʡ�ԣ�������������λ��Ч���֣�������Һ��c(H2C2O4)________c(C2O42-)���С�ڡ��������ڡ����ڡ�����
���𰸡� ̽���¶ȼ���Ӧʱ��������������ʵ�Ӱ�� С�� �÷�ӦΪ���ȷ�Ӧ�������¶�ƽ�������ƶ� ����̼���� ���� ���� 2MnO4-+5H2C2O4+ 6H+= 2Mn2++ 10CO2��+ 8H2O 1.7��10-13 С��
����������1��������ͼ��֪����ʵ���ʵ��Ŀ���ǣ�̽����Ӧ�¶ȡ���Ӧʱ��������������ʵ�Ӱ�죻�������������IJ��ʺ�ʱ��ı�ֵ֪��60���·�Ӧ40min��70���·�Ӧ20min��ȣ�ǰ�ߵ�ƽ����Ӧ����С�ں��ߣ�
���Ҵ���������лӷ��ԣ�����������ˮ�ⷴӦ�����ȷ�Ӧ������Ӧ�ﵽƽ��״̬ʱ���������¶ȣ��ٽ���������ʱ�䣬ƽ�����淴Ӧ�����ƶ����¶ȹ��ߣ��Ҵ�����������ӷ�ʹ��Ӧ���������½���
�����������ڱ���̼������Һ���ܽ�Ƚ�С�������ᡢ�Ҵ��Լ�������������ܽ����л����䷴Ӧ��ת��Ϊ������ˮ���Σ����ᴿʱ�����Ʒ�м��뱥��̼������Һ���г��ϴ�ӣ���������л��������ˮ�����Ƹ����ȥˮ�ݣ�ͨ�����˳�ȥ�����ƾ��塢�����ɵõ���Ʒ��
��2�������ᱻ����KMnO4��Һ��������CO2���壬ͬʱKMnO4����ԭΪMn2+�����ݵ����غ㡢����غ㼰ԭ���غ�ò���������KMNO4��Һ��Ӧ�����ӷ���ʽ2MnO4-+5H2C2O4+ 6H+= 2Mn2++ 10CO2��+ 8H2O��
��HC2O4-��ˮ�ⷽ��ʽΪ��HC2O4-+H2OH2C2O4+OH-��HC2O4-��ˮ��ƽ�ⳣ��Ϊ��Kh=![]() =
=![]() =1.7��10-13���ɴ˿ɼ�����Һ��HC2O4-��ˮ��С������룬��c(H2C2O4)С��c(C2O42-)��
=1.7��10-13���ɴ˿ɼ�����Һ��HC2O4-��ˮ��С������룬��c(H2C2O4)С��c(C2O42-)��