��Ŀ����
����Ŀ���������ѧ֪ʶ�ش��������⣺
�� | ���볣�� |
CH3COOH | K = 1.8��10 -5 |
H2CO3 | K1= 4.3��10 -7��K2= 5.6��10 -11 |
H2SO3 | K1=1.54��10-2 ��K2=1.02��10-7 |
��1��NaHSO3��Һ�й�����7������������Na+��HSO3-��H+��SO32-��H2O��________��_________���������ţ���
��2�������£����ʵ���Ũ����ͬ��������Һ ��
��NH4Cl �� NH4HCO3 �ۣ�NH4��2SO4 �� NH4HSO4
��Һ��c(NH4+)�����ǣ�_________����С���ǣ�______������ţ�
��3�������£����ʵ���Ũ�Ⱦ�Ϊ0��1mol/L��������Һ��NaOH����NaCl����Na2CO3����H2SO3����CH3COONa����H2SO4��pH�Ӵ�С����˳��Ϊ___________������ţ�
��4������ʱ��AlCl3��ˮ��Һ�����ԣ�ԭ���ǣ������ӷ���ʽ��ʾ����_____________��AlCl3��Һ���ɣ����գ����õ��Ĺ��������Ҫ��________���ѧʽ��
���𰸡� OH- H2SO3 �� �� �٣��ۣ��ݣ��ڣ��ܣ��� Al3+�� 3H2O ![]() Al (OH)3 �� 3H+ Al2O3
Al (OH)3 �� 3H+ Al2O3
��������(1)NaHSO3��ǿ�������Σ�����ȫ����NaHSO3�TNa++HSO3-��������Һ�д���Na+��HSO3-����Һ�л�����HSO3-����HSO3-H++SO32-��������Һ�д���H+��SO32-��Ҳ����HSO3-ˮ��HSO3-+H2OH2SO3+OH-��������Һ�д���H2SO3��OH-������NaHSO3��Һ�й�����7������������Na+��HSO3-��H+��SO32-��H2O��OH-��H2SO3���ʴ�Ϊ��OH-��H2SO3��
(2)�����£����ʵ���Ũ����ͬ�Ģ�NH4Cl���� NH4HCO3����(NH4)2SO4���� NH4HSO4�Т���c(NH4+)���ԼΪ�����2��������̼���������ˮ��ٽ�笠����ӵ�ˮ�⣬����c(NH4+)��С���ʴ�Ϊ����������
(3)��NaOH����NaCl����Na2CO3����H2SO3����CH3COONa����H2SO4����Ϊǿ�pH���Ϊǿ�ᣬpH��С����Ϊǿ��ǿ���Σ�pH=7���ۢ�Ϊ����ǿ���Σ�ˮ���Լ��ԣ������ԱȢ�������Ϊ���ᣬ����pH�Ӵ�С����˳��Ϊ�����������������������ޣ��ʴ�Ϊ��������������������������
(4)AlCl3Ϊǿ����ʣ�����Һ����ȫ���룬����Al3+���Ӻ�Cl-���ӣ����뷽��ʽΪAlCl3�TAl3++3Cl-��Al3+ˮ�����ӷ���ʽΪ��Al3++3H2OAl(OH)3+3H+��AlCl3��Һ�����ԣ���AlCl3��Һ���ɣ��Ȼ���ӷ����ٽ�ˮ�����������������������յõ����������ʴ�Ϊ��Al3++3H2OAl(OH)3+3H+��Al2O3��

����Ŀ����͵��ĵ��ʼ�һЩ�������ڹ�ũҵ��������������ҪӦ�á��ش�����������
��1��Nԭ�Ӻ�����___�ֲ�ͬ�˶�״̬�ĵ��ӡ���̬Nԭ���У�������ߵĵ�����ռ�ݵ�ԭ�ӹ������״Ϊ___________��
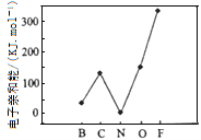
��2��Ԫ�صĻ�̬��̬ԭ�ӵõ�һ�������γ���̬��һ������ʱ���ų�������������һ�������ܣ�E1�����ڶ����ڲ���Ԫ�ص�E1�仯������ͼ��ʾ�����г���Ԫ���⣬����Ԫ�ص�E1����������������ԭ����_______________________����Ԫ�ص�E1�����쳣��ԭ����_______________________��
��3�����ⶨ���֣�N2O5������NO2+��NO3-����������ɣ��ù�����Nԭ���ӻ�����Ϊ___________________��
��4����δ��ȶ���NH4F��NH4I�У����ֽ����____��ԭ����__________________��
��5���ڶ������У���һ�����ܽ���BԪ�غ�NԪ�ؼ��Ԫ��Ϊ_____(����Ԫ�ط�����)��
��6����֪����NO2 + CO ![]() CO2 + NO
CO2 + NO
ÿ1mol�������ʷֽ�Ϊ��̬��̬ԭ�����������ֱ�Ϊ
NO2 | CO | CO2 | NO |
812kJ | 1076kJ | 1490kJ | 632kJ |
��N2(g)+O2(g) ![]() 2NO(g) ��H��+179.5 kJ/mol
2NO(g) ��H��+179.5 kJ/mol
��2NO(g) +O2(g)![]() 2NO2(g) ��H��-112.3 kJ/mol
2NO2(g) ��H��-112.3 kJ/mol
��д��NO��CO��Ӧ��������Ⱦ��������Ȼ�ѧ����ʽ_________________��
����Ŀ��ʵ��������450 mL3.0 mol��L��1 H2SO4��Һ��ijͬѧ��98%��Ũ���� ���ܶ�Ϊ1.84 g/mL�������в�����������
��1����ش��й����⡣
ʵ�鲽�� | �й����� |
a.��������ŨH2SO4������� | ��ȡŨH2SO4�����Ϊ��__________ |
b.��ȡŨ������ | |
c.��ŨH2SO4�������뵽װ������ˮ��200 mL�ձ����� | |
d.���ձ��е���Һ��ȴ��ת������__________���� | |
e.ϴ�Ӻ�������ƿ�м�����ˮ��̶���1��2cm�����â�__________������ |
��2������H2SO4��Һʱ�����������в���������ƫ����ƫ�ͣ�
A����ȡŨH2SO4��Һʱ���Ӷ��� ____��
B������ʱ���ӿ̶��� ___��
C��Ũ��������ˮ��δ����ȴ��ת��____��




