��Ŀ����
8�������Ϊ2L�������м���1mol N2��6mol H2���п��淴Ӧ��N2+3H2$?_{��}^{����}$2NH3����Ӧ2min���N2�����ʵ���Ϊ0.6mol������1��2min�ڣ�H2�����ʵ���������1.2mol��NH3�����ʵ���������0.8mol��
��2������N2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ���ʣ���v��N2��=0.1mol/��L•min����
��3������NH3��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ���ʣ���v��NH3��=0.2mol/��L•min����
���� ��1�����ݻ�ѧƽ�������ʽ��ʽ����õ�2min������ʵ������Ӷ�����H2��NH3�����ʵ����ı仯����
��2������3������v=$\frac{��c}{��t}$����v��N2����v��NH3����
��� �⣺��1�������Ϊ2L�������м���1mol N2��6mol H2���п��淴Ӧ��N2+3H2$?_{��}^{����}$2NH3����Ӧ2min���N2�����ʵ���Ϊ0.6mol����
N2 +3H2$?_{��}^{����}$ 2NH3
��ʼ����mol��1 6 0
�仯����mol��0.4 1.2 0.8
ƽ������mol��0.6 4.4 0.8
��1��2min�ڣ�H2�����ʵ���������1.2mol��NH3�����ʵ���������0.8mol��
�ʴ�Ϊ��1.2mol��0.8mol��
��2����N2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ����v��N2��=$\frac{\frac{��n}{V}}{��t}$=$\frac{\frac{0.4mol}{2L}}{2min}$=0.1mol/��L•min����
�ʴ�Ϊ��0.1mol/��L•min����
��3����NH3��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ����v��NH3��=$\frac{\frac{��n}{V}}{��t}$=$\frac{\frac{0.8mol}{2L}}{2min}$=0.2mol/��L•min����
�ʴ�Ϊ��0.2mol/��L•min����
���� ���⿼���˻�ѧƽ��ļ���Ӧ�ã�ƽ������ʽ���㷽���ǽ���ؼ�����Ŀ�ϼ�

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�| A�� | �Ҵ������ᡢ�����������ܷ���ȡ����Ӧ�����������е�����������ñ���Na2CO3��Һ��ȥ | |
| B�� | �������ۡ�ֲ���͡������ʶ����ɸ߷��ӻ�������ɵ����ʣ�����ˮ�⣬��ˮ����ﲻͬ | |
| C�� | ���顢��ϩ���Ҵ������ụΪͬϵ�����������ǻ�Ϊͬ���칹�� | |
| D�� | ��ϩ������ϩ�ͱ�����ʹ��ˮ��ɫ����ɫ��ԭ����ͬ |
| A�� | �Ȼ�����Һ���ռ���Һ�������� | B�� | �ռ���Һ����ɫʯ����Һ | ||
| C�� | ����Cu��OH��2����Һ����ɫʯ����Һ | D�� | ����Cu��OH��2����Һ����ˮ |
| A�� | �������ڿ�����ȼ�գ�����ɫ����ɫ | |
| B�� | ����˿�ڴ�����ȼ�գ���ɫ����ɫ | |
| C�� | ��������SO2�ķ�̪��Һ����ɫ����ɫ | |
| D�� | ������NO�Ӵ���������ɫ������ɫ |
��2��CO2����Ȼ��ѭ��ʱ����CaCO3��Ӧ��CaCO3��һ���������ʣ���Ksp=3.8��10 -9��CaCl2��Һ��Na2CO3��Һ��Ͽ��γ�CaCO3�������ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ���Na2CO3��Һ��Ũ��Ϊ4��10 -4mo1/L�������ɳ�������CaCl2��Һ����СŨ��Ϊ3.8��10-5mol/L��
�����¶�t���£�ijBa��OH��2��ϡ��Һ��c��H+��=10-amol/L��c��OH-��=10-bmol/L����֪a+b=12�������Һ����μ���pH=b��NaHSO4����û����Һ�IJ���pH���±���ʾ��
| ��� | ����������Һ�����/mL | ����������Һ�����/mL | ��Һ��pH |
| �� | 33.00 | 0.00 | 8 |
| �� | 33.00 | x | 7 |
| �� | 33.00 | 33.00 | 6 |
��2�������¶��µ�Ba��OH��2��Һȡ��1ml����ˮϡ����1L�����ϡ�ͺ����Һ��c��Ba2+���sc��OH-��=1��20��
��3����NaHSO4��ͬ��NaHSO3 ��NaHCO3ҲΪ��ʽ�Σ���֪NaHSO3��Һ�����ԡ�NaHCO3��Һ�ʼ��ԣ�����Ũ�Ⱦ�Ϊ0.1mol•L-1��NaHSO3��Һ��NaHCO3��Һ����Һ�и����ӵ����ʵ���Ũ�ȴ������й�ϵ��R��ʾS��C�������п�����ȷ����AC������ĸ��
A��c��Na+����c��HRO3-����c��H+����c��RO32-����c��OH-��
B��c��Na+��+c��H+��=c��HRO3-��+c��RO32-��+c��OH-��
C��c��H+��+c��H2RO3��=c��RO32-��+c��OH-��
D������Һ��c��Na+����c��HRO3-����c��RO32-���ֱ���ȣ�
| A�� | �����¶� | B�� | ��Zn�۴���Zn�� | ||
| C�� | ����0.1mol/LH2SO4��Zn��Ӧ | D�� | �μ�������CuSO4��Һ |
| A�� | �û���̿ȥ�������е���ζ | |
| B�� | �ý��ݹ����������Һ�Ĺ���������ˮ�� | |
| C�� | ���ȼ�ˮ��������ϲ��������� | |
| D�� | �ú��轺�����۵���С����ʳƷһ���ܷ��װ |
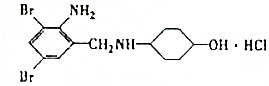
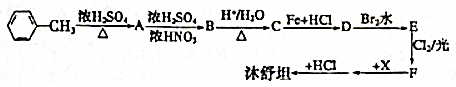
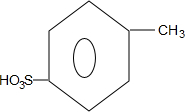 ��
�� ��
��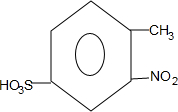 +H2O��
+H2O��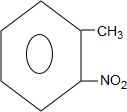 +H2SO4��
+H2SO4��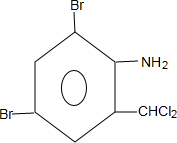 +2NaOH��
+2NaOH��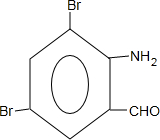 +2NaCl+H2O��д��H����������Ӧ�����ӷ���ʽ
+2NaCl+H2O��д��H����������Ӧ�����ӷ���ʽ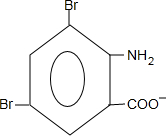 +NH4++2Ag��+3NH3+H2O��
+NH4++2Ag��+3NH3+H2O��