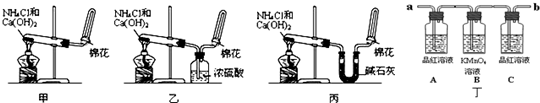
��Ŀ����
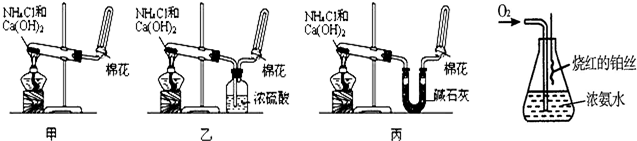
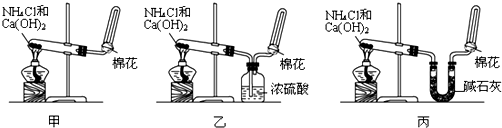
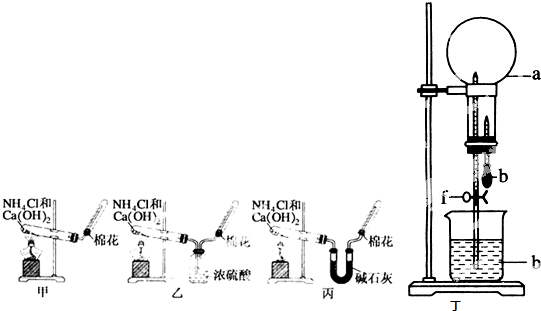
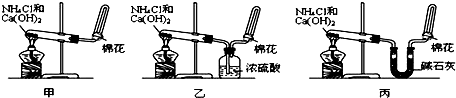
�ס��ҡ�����λͬѧ�ֱ�������ʵ��װ�ü���ѧҩƷ����ʯ��Ϊ�������ƺ���ʯ�ҵĻ�����ȡ�������������̽�������ش����⣺��1����ȡ�����Ļ�ѧ����ʽΪ��______��
��2����λͬѧ���������ſ������ռ���������ԭ����______��
��3����λͬѧ������װ����ȡ����ʱ��������һλͬѧû���ռ�������ʵ���������ȷ��������Ϊû���ռ���������ͬѧ��______����ס������ҡ���������
��4�����鰱���Ƿ��ռ����ķ����ǣ�������������������ͽ��ۣ���______��
��5����λͬѧ����Ϊ�������������Ե�װ�ã��������ڼ���̼����粒���ķ�������ȡ�����İ���������Ϊ��λͬѧ�ܹ��ﵽʵ��Ŀ��______����ס������ҡ������������ǻ���Ϊ��װ���е�NH4HCO3�������NH4Cl������棬����Ϊ______����ܡ����ܡ�����
��6��ij����С������й�����֪����˿�ǰ������Ĵ���������ƽ�������ͼʵ�飮ʵ������й۲쵽��ƿ�������Ϊ����ɫ��ƿ�ڳ��ְ���
д����ʵ������л�ѧ��Ӧ����ʽ��______���¸�ѹ4NO+6H2O
���𰸡���������1��ʵ�������������ƺ��Ȼ���ڼ����������Ʊ���������Ӧ����ʽΪ2NH4Cl+Ca��OH��2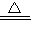 CaCl2+2H2O+2NH3��
CaCl2+2H2O+2NH3��
��2�����ݰ������ܽ��Ժ��ܶ�ѡ���ռ������ķ�����
��3�������������ᷴӦ�����������գ�
��4������Ϊ�������壬��ˮ��Ӧ����NH3?H2O�������ӳ�OH-���ӣ���Һ�ʼ��ԣ�
��5��̼����識��ȷֽ����ɰ�����������̼��ˮ�����ü�ʯ�ҳ��ӣ���������NH4HCO3�������NH4Cl���壻
��6�����ݷ�Ӧ��������ʵ�������д��ѧ����ʽ��
����⣺��1��ʵ�������������ƺ��Ȼ���ڼ����������Ʊ���������Ӧ����ʽΪ2NH4Cl+Ca��OH��2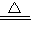 CaCl2+2H2O+2NH3����
CaCl2+2H2O+2NH3����
�ʴ�Ϊ��2NH4Cl+Ca��OH��2�TCaCl2+2NH3��+2H2O��
��2������������ˮ����������ˮ���ռ��������ܶȱȿ���С��Ӧ�������ſշ��ռ���
�ʴ�Ϊ���������ܶȱȿ���С��������������ˮ��
��3������Ϊ�������壬ͨ��ʢ��Ũ�����ϴ��ƿʱ�������ᷴӦ�������գ��ʴ�Ϊ���ң�
��4������Ϊ�������壬��ˮ��Ӧ����NH3?H2O�������ӳ�OH-���ӣ���Һ�ʼ��ԣ�����ʱ������պ��Ũ����IJ����������Թܿڣ�����������̣�
��������������ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ������ֽ����������������
�ʴ�Ϊ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ������ֽ����������������
��5��̼����識��ȷֽ����ɰ�����������̼��ˮ�����п��ü�ʯ�����ն�����̼��ˮ��ֻ�б����������Ȼ�識��ȷֽ����ɰ������Ȼ��⣬��
�¶Ƚϵ�ʱ�����������Ȼ�泥�����ֻ���Ȼ���Ʊ��������ʴ�Ϊ���������ܣ�
��6�������ڴ����������¼��ȷ�Ӧ����NO��ˮ��NO�ױ��������ɺ���ɫ��NO2����һ����ˮ��Ӧ�������ᣬ���õ��İ���������泥���Ӧ
���йط���ʽΪ4NH3+5O2 4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��NH3+HNO3�TNH4NO3��
4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��NH3+HNO3�TNH4NO3��
�ʴ�Ϊ��4NH3+5O2 4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��NH3+HNO3�TNH4NO3��
4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��NH3+HNO3�TNH4NO3��
���������⿼�鰱��ʵ��̽������Ŀ�Ѷ��еȣ�ע��ʵ�����Ʊ�����������ҩƷ��
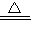 CaCl2+2H2O+2NH3��
CaCl2+2H2O+2NH3����2�����ݰ������ܽ��Ժ��ܶ�ѡ���ռ������ķ�����
��3�������������ᷴӦ�����������գ�
��4������Ϊ�������壬��ˮ��Ӧ����NH3?H2O�������ӳ�OH-���ӣ���Һ�ʼ��ԣ�
��5��̼����識��ȷֽ����ɰ�����������̼��ˮ�����ü�ʯ�ҳ��ӣ���������NH4HCO3�������NH4Cl���壻
��6�����ݷ�Ӧ��������ʵ�������д��ѧ����ʽ��
����⣺��1��ʵ�������������ƺ��Ȼ���ڼ����������Ʊ���������Ӧ����ʽΪ2NH4Cl+Ca��OH��2
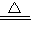 CaCl2+2H2O+2NH3����
CaCl2+2H2O+2NH3�����ʴ�Ϊ��2NH4Cl+Ca��OH��2�TCaCl2+2NH3��+2H2O��
��2������������ˮ����������ˮ���ռ��������ܶȱȿ���С��Ӧ�������ſշ��ռ���
�ʴ�Ϊ���������ܶȱȿ���С��������������ˮ��
��3������Ϊ�������壬ͨ��ʢ��Ũ�����ϴ��ƿʱ�������ᷴӦ�������գ��ʴ�Ϊ���ң�
��4������Ϊ�������壬��ˮ��Ӧ����NH3?H2O�������ӳ�OH-���ӣ���Һ�ʼ��ԣ�����ʱ������պ��Ũ����IJ����������Թܿڣ�����������̣�
��������������ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ������ֽ����������������
�ʴ�Ϊ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ������ֽ����������������
��5��̼����識��ȷֽ����ɰ�����������̼��ˮ�����п��ü�ʯ�����ն�����̼��ˮ��ֻ�б����������Ȼ�識��ȷֽ����ɰ������Ȼ��⣬��
�¶Ƚϵ�ʱ�����������Ȼ�泥�����ֻ���Ȼ���Ʊ��������ʴ�Ϊ���������ܣ�
��6�������ڴ����������¼��ȷ�Ӧ����NO��ˮ��NO�ױ��������ɺ���ɫ��NO2����һ����ˮ��Ӧ�������ᣬ���õ��İ���������泥���Ӧ
���йط���ʽΪ4NH3+5O2
 4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��NH3+HNO3�TNH4NO3��
4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��NH3+HNO3�TNH4NO3���ʴ�Ϊ��4NH3+5O2
 4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��NH3+HNO3�TNH4NO3��
4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��NH3+HNO3�TNH4NO3�����������⿼�鰱��ʵ��̽������Ŀ�Ѷ��еȣ�ע��ʵ�����Ʊ�����������ҩƷ��

��ϰ��ϵ�д�
�����Ŀ