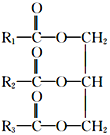
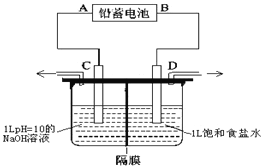
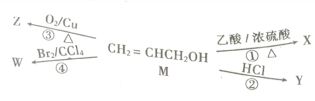��Ŀ����
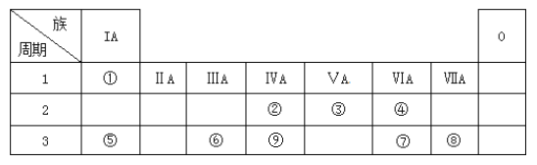
����Ŀ���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺
��1������������Ԫ�طǽ�������ǿ��Ԫ�ص�ԭ�ӽṹʾ��ͼΪ__________��
��2���ڢۢ�����������Ӧˮ��������ǿ��˳��Ϊ���ѧʽ��_____________��
��3���õ���ʽ��ʾ�ܵ��⻯����γɹ���_________________________��
��4�����п����жϢݺ͢�����ǿ������__________��
a. �ݵ��ʵ��۵�Ȣ��ʵ�
b. �ݵĻ��ϼ۱Ȣ�
c. �ݵ�����ˮ��Ӧ�ȵ��ʢ���
d. ������������ˮ����ļ��ԱȢ�ǿ
��5���ɱ��Т١��ۡ��ܡ��ޡ���Ԫ���γɵij�������Z��M��N�ɷ������·�Ӧ��
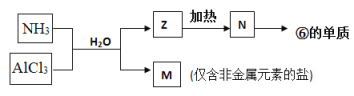
a. M�������Ļ�ѧ������Ϊ���������ۼ�����������Ի�Ǽ��ԣ�_________��
b. N���ĵ��ʵĻ�ѧ����ʽ_________________________________________��
���𰸡� HNO3��H2CO3��H2SiO3
HNO3��H2CO3��H2SiO3![]() cd���Թ��ۼ������Ӽ�2Al2O3�����ڣ�
cd���Թ��ۼ������Ӽ�2Al2O3�����ڣ�![]() 4Al+O2��
4Al+O2��
��������
��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪS����ΪCl����ΪSi����
��1��ͬ�����������ҷǽ���������ǿ�������������Ԫ�طǽ�������ǿ��Ԫ������Ԫ�أ���ԭ�ӽṹʾ��ͼΪ ��
��
��2���ǽ�����Խǿ����ۺ����������Խǿ����ڢۢ�����������Ӧˮ��������ǿ��˳��ΪHNO3��H2CO3��H2SiO3��
��3���ܵ��⻯����ˮ�����õ���ʽ��ʾ���γɹ���Ϊ![]() ��
��
��4��a. ������ǿ����������ʵ��۵�ߵ�û�й�ϵ��a����
b. ������ǿ�������Ԫ�صĻ��ϼ۸ߵ�û�й�ϵ��b����
c. ������Խǿ���䵥��Խ������ˮ��Ӧ����ݵ�����ˮ��Ӧ�ȵ��ʢ���˵���ƵĽ�����ǿ������c��ȷ��
d. ������Խǿ�����������ˮ����ļ���Խǿ���������������ˮ����ļ��ԱȢ�ǿ˵���ƵĽ�����ǿ������d��ȷ��
��ѡcd��
��5������ͨ���Ȼ�����Һ���������������������Ȼ�泥���M���Ȼ�泥�Z���������������������ֽ�����N����������������ڵ��������õ�����������
a. �Ȼ���������Ļ�ѧ�����������Ӽ��ͼ��Թ��ۼ���
b. ���ұ�����Ļ�ѧ����ʽΪ2Al2O3�����ڣ�![]() 4Al+O2����
4Al+O2����

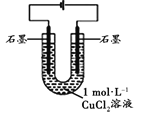
����Ŀ��ijС��ͬѧ������ͼװ�öԵ���Ȼ�ͭʵ��������о���
װ�� | ���� |
| ���һ��ʱ��ʱ������ʯī����������壬����ʯī�ϸ��ź�ɫ���ʣ��ձ��ڱ��ȣ���Һ����ɫ��Ϊ��ɫ |
��1������Ϊ������������������_________����Һ���̵�ԭ��
��2���Ҳ������ϣ�CuCl2��Һ�д���ƽ�⣺Cu2+ + 4Cl-![]() [CuCl4]2-(��ɫ) ��H��0���ݴ�����Ϊ:�������У�[CuCl4]2-(��ɫ)Ũ��������CuCl2��ɫ��Һ��ϳ���ɫ��������ƽ���ƶ�ԭ���Ʋ��ڵ�������[CuCl4]2-Ũ�������ԭ��________________��
[CuCl4]2-(��ɫ) ��H��0���ݴ�����Ϊ:�������У�[CuCl4]2-(��ɫ)Ũ��������CuCl2��ɫ��Һ��ϳ���ɫ��������ƽ���ƶ�ԭ���Ʋ��ڵ�������[CuCl4]2-Ũ�������ԭ��________________��
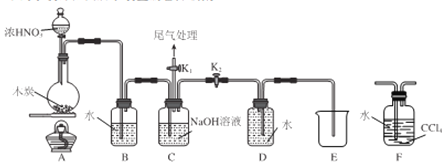
��3����������ͼװ�ã�����ͬ�����µ��CuCl2��Һ������Һ��ɫ�������̽����
װ�� | ���� |
| �����ͬʱ��ʱ������ʯī����������ݣ���Һ��Ϊ��ɫ������ʯī�ϸ��ź�ɫ���ʣ���Һ����ɫ��Ϊ��ɫ��U�ܱ��ȣ���ȴ������������Һ��Ϊ��ɫ |
��ͨ�����������֤ʵ�˼��ҵĹ۵��������Һ���̵���Ҫԭ����������________�����ҵ�������________________��
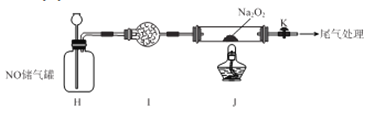
��4���������������ϣ�
i. ���CuCl2��Һʱ���ܲ���[CuCl2]-��[CuCl2]-����Cu2+��ʻ�ɫ
ii. ϡ�ͺ�[CuCl2]-����Һ����CuCl��ɫ�����ݴ˱���Ϊ���������У�����[CuCl2]-����Cu2+��ʻ�ɫ����CuCl2��ɫ��Һ��ϳ���ɫ��
����������ʵ�飺
a��ȡ������ɫ��Һ2 mL����20 mLˮϡ�ͣ�����5���Ӻ���Һ�в�����ɫ������
b. ��ȡ�����Ȼ�ͭ�����ͭ�ۣ������м�2 mLŨ���ᣬ���Ȼ�ú�[CuCl2]-�Ļ�ɫ��Һ��
c. ��ȴ����������Һ����
d. ȡc��2 mL��Һ����20 mLˮϡ�ͣ�����5���Ӻ���Һ�в�����ɫ������
�� a��Ŀ����__________________��
�� д��b������[CuCl2]-�����ӷ���ʽ��____________________��
�� ����c�б�Ҫ�IJ���������____________________��
���ݴ˵ó����ۣ����ʱ������������[CuCl2]-�ǵ�����Һ���̵�ԭ��