��Ŀ����
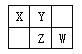
����Ŀ���±���Ԫ�����ڱ���һ���֡�����Ԫ���ڱ��е�λ�ã����û�ѧ����ش���������:
�� ���� | IA | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
1 | �� | |||||||
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | ||||
4 | �� | |||||||
5 | �� |
��1�������ۺ�����Ļ�ѧʽΪ_________��
��2���ٺ͢�����Ԫ�ص�ԭ�Ӱ�1:1��ɵij���������ĽṹʽΪ_________��
��3���ۢܢߢ�ļ�����뾶�ɴ�С��˳��Ϊ_________��(�����ӷ��ű�ʾ)
��4���ڢܵ�����������ˮ����֮�䷢����Ӧ�����跽��ʽ_________��
��5���õ���ʽ��ʾ�ۺ͢���ɵĻ�������γɹ���_________��
��6�������к��Т�Ԫ�أ��������к��и�Ԫ�صļ������ӡ��������ữ�£�����˫��ˮ��������Ϊ���ʡ�д���÷�Ӧ�����ӷ���ʽ_________��
���𰸡� HBrO4 H-O-O-H Cl->O2->Mg2+>Al3+ Al(OH)3+OH-=AlO2-+2H2O 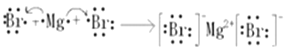 H2O2+2I-+2H+=2H2O+I2��
H2O2+2I-+2H+=2H2O+I2��
����������Ԫ�������ڱ���λ�ã���֪��ΪH����ΪNa����ΪMg����ΪAl����ΪC����ΪN����ΪO����ΪCl����ΪBr����ΪI��
��1����ΪBr��Br���������Ϊ+7��������ۺ�����ΪHBrO4��
�ʴ�Ϊ�� HClO4��
��2���ٺ͢�����Ԫ�ص�ԭ�Ӱ�1:1��ɵij���������ΪH2O2����ṹʽΪH-O-O-H��
�ʴ�Ϊ��H-O-O-H��
��3���ۢܢߢ�ļ����ӷֱ�Ϊ��Mg2+��Al3+��O2-��Cl-��ǰ�������ӵĺ�������Ų���ͬ����������İ뾶����С��Cl-���������Ӳ㣬�ʰ뾶���
�ʴ�Ϊ��Cl->O2->Mg2+>Al3+��
��4���ڢܵ�����������ˮ����ֱ�ΪNaOH��Al(OH)3,���߷�Ӧ�����ӷ���ʽΪ��
Al(OH)3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al(OH)3+OH-=AlO2-+2H2O
��5���ۺ͢��γɵĻ�����ΪMgBr2�����ӻ�������õ���ʽ��ʾ���γɹ������£�![]() ��
��
��6�������к���IԪ�أ��������к���I-���������ữ�Ļ����£���˫��ˮ�ɽ�������Ϊ�ⵥ�ʡ��ʴ�Ϊ��H2O2+2I-+2H+=2H2O+I2��

 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�