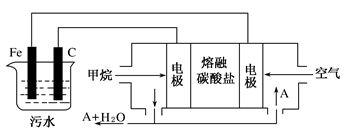
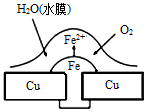
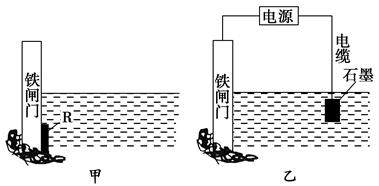
��Ŀ����
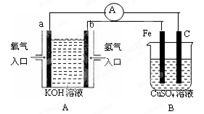
����ʵ��װ���У�ʵ��ʱ���ȶϿ�K2���պ�K1�������������ݲ�����һ��ʱ��Ͽ�K1���պ�K2�����ֵ�����ָ��ƫת�������й�������ȷ����( )
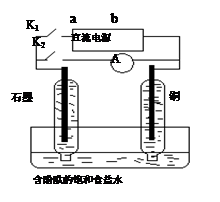

A���Ͽ�K2���պ�K1ʱ���ܷ�Ӧ�����ӷ���ʽΪ��2H++2Cl�� Cl2��+H2�� Cl2��+H2�� |
| B���Ͽ�K2���պ�K1ʱ�������ء�b ��Cu���������Һ��ʯī��a����·������ |
| C���Ͽ�K2���պ�K1ʱ��ͭ�缫������Һ��� |
| D���Ͽ�K1���պ�K2ʱ��ͭ�缫�ϵĵ缫��ӦΪ��Cl2+2e����2Cl�� |
C
���������A���Ͽ�K2���պ�K1ʱ�����������ݲ������ܷ�Ӧ�����ӷ���ʽΪ��2Cl?+2H2O
 Cl2��+H2��+2OH?������B�������������ݲ�����ͭ�缫Ϊ����������b�缫Ϊ��Դ�ĸ��������ǵ��Ӳ����ڵ��Һ������������C���Ͽ�K2���պ�K1ʱ��ͭ�缫Ϊ������H2O�������H+�ŵ磬ʹH2O�ĵ���ƽ�������ƶ���OH?Ũ������ʹ��̪��죬��ȷ��D���Ͽ�K1���պ�K2ʱ��Cl2��H2��������Һ�γ�ԭ��أ�ͭ�缫��H2�ŵ磬����
Cl2��+H2��+2OH?������B�������������ݲ�����ͭ�缫Ϊ����������b�缫Ϊ��Դ�ĸ��������ǵ��Ӳ����ڵ��Һ������������C���Ͽ�K2���պ�K1ʱ��ͭ�缫Ϊ������H2O�������H+�ŵ磬ʹH2O�ĵ���ƽ�������ƶ���OH?Ũ������ʹ��̪��죬��ȷ��D���Ͽ�K1���պ�K2ʱ��Cl2��H2��������Һ�γ�ԭ��أ�ͭ�缫��H2�ŵ磬����
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ