��Ŀ����
11��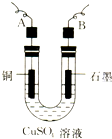 ijѧ���õ�ⴿ����CuSO4��Һ�ķ����������ݵ缫������Cu��������m g���Լ��缫�ϲ�������������V mL ��״�������ⶨCu�����ԭ�����������ò�����������ͼ��ʾ���ش��������⣺
ijѧ���õ�ⴿ����CuSO4��Һ�ķ����������ݵ缫������Cu��������m g���Լ��缫�ϲ�������������V mL ��״�������ⶨCu�����ԭ�����������ò�����������ͼ��ʾ���ش��������⣺��1���������ӷ���ʽΪ2Cu2++2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+4H+��
��2������ʵ������б�Ҫ����A��B��D��E����д��ĸ����
��A���������ǰ�ĵ缫��������
��B�����缫�ں�ɳ���ǰ������������ˮ��ϴ��
��C�����µ���缫��������ͭ������ϴ��������
��D�������ɳ��صIJ����б��밴����ɡ��������ٺ�ɡ��ٳ��������У�
��E�����п������ڵ�����£���ɵ缫�����õ��º�ɵķ�����
��3��ͭ�����ԭ������Ϊ$\frac{11200m}{V}$���ô���m��V�ļ���ʽ��ʾ����
���� ��1���������ͭ��Һ����������������ʧ���ӣ�����ͭ���ӵõ��ӣ�
��2�����ݵ缫������ͭ�������Լ��缫�ϲ��������������ⶨͭ�����ԭ������������Ҫ�������ǰ��缫��������
��3�����ݵ����غ��֪��2Cu��02�����Դ˼��㣮
��� �⣺��1���������ͭ��Һ����������������ʧ���ӣ�����ͭ���ӵõ��ӣ����ⷴӦ�����ӷ���ʽΪ2Cu2++2H20$\frac{\underline{\;���\;}}{\;}$2Cu+4H++02����
�ʴ�Ϊ��2Cu2++2H20$\frac{\underline{\;���\;}}{\;}$2Cu+4H++02����
��2�����ݵ缫������ͭ�������Լ��缫�ϲ��������������ⶨͭ�����ԭ������������Ҫ�������ǰ��缫��������
A��ʵ��֮ǰӦ�������ǰ�缫������������ȷ
B�����缫�ں�ɳ���ǰ������������ˮ��ϴ������������ȷ��
C�����µ���缫��������ͭ������ϴ�����أ���������ȷ���ѵõ�ȷ��Cu���������ʴ���
D���缫�ں�ɳ��صIJ����б��밴�����-����-�ٺ��-�ٳ��ء����У���ֹCu������������ȷ��
E�����п������ڵ�����£���ɵ缫������õ��º�ɵķ�������ֹCu������������ȷ��
�ʴ�Ϊ��ABDE��
��3����Cu�����ԭ������Ϊx��
���ݵ����غ��֪��2Cu��02����
2x 1
ng $\frac{V��1{0}^{-3}L}{22.4L/mol}$
�� 2x��$\frac{V��1{0}^{-3}L}{22.4L/mol}$=n��
��ã�x=$\frac{11200m}{V}$��
�ʴ�Ϊ��$\frac{11200m}{V}$��
���� ���⿼���˵��ԭ����Ӧ�ü����ԭ�������IJⶨ��ע�����е����غ㼴�ɽ����Ŀ�Ѷ��еȣ������ڿ���ѧ���ķ��������ͼ���������

 ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�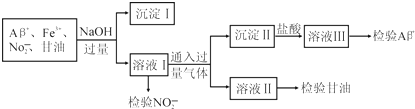
��֪��Al3++6F-�T[AlF6]3-��
| A�� | ͨ��Ĺ������������CO2 | |
| B�� | �����������IJ��������ǹ��ˣ����ó�����ɼ����Fe3+ | |
| C�� | ������Һ�����ȼ�����NH4F���ټӰ�ˮ���Լ���Al3+ | |
| D�� | ������KI��Һ����NO2-���ӵ����ӷ���ʽΪ2NO2-+2I-+4H+�T2NO+I2+2H2O |
| A�� | pH=2��HA��Һ��pH=12��MOH��Һ������Ȼ��c��H+��+c��M+��=c��OH-��+c��A-�� | |
| B�� | Na2CO3��Һ��c��OH-��=c��HCO3-��+c��H+��+2c��H2CO3�� | |
| C�� | ������pH=7��CH3COOH��CH3COONa�Ļ��Һ�����ӵ�Ũ�ȴ�С˳��Ϊ��c��Na+��=c��CHCOO-����c��H+��=c��OH-�� | |
| D�� | pH=4��NaHA��Һ��c��HA-����c��H+����c��H2A����c��A2-�� |
| A�� | �ƾ� | B�� | ���� | C�� | ���Ȼ�̼ | D�� | ���� |
| A�� | ��Һ�е�SO42-�������˶� | B�� | ����ͨ��������ͭƬ����пƬ | ||
| C�� | ������O2�ݳ� | D�� | ͭƬ����H2�ݳ� |
| A�� | �����ʯ��ˮ��ϡ���ᷴӦ��OH-+H+=H2O | |
| B�� | ����������Һ��ϡ���ᷴӦ��Ba2++OH-+SO${\;}_{4}^{2-}$+H+=BaSO4��+H2O | |
| C�� | ����ͭ�����ᷴӦ��O2-+2H+=H2O | |
| D�� | ������ˮ��Ӧ��Cl2+H2O=2H++Cl-+ClO- |
| A�� | Na+��Mg2+��Al3+ | B�� | HCl��H2S��Ar | C�� | H2O��OH-��Na+ | D�� | NH4+��Na+��F- |
| A�� | ��ˮ���۲��Ƿ��зֲ����� | |
| B�� | ���Ҵ����۲��Ƿ����ֲ� | |
| C�� | ������Cu��OH��2����Һ����У��۲�����ש��ɫ�������� | |
| D�� | ���뺬�з�̪��NaOH��Һ�����ȣ��۲��ɫ�Ƿ��dz |