��Ŀ����
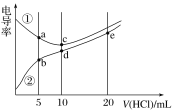
����Ŀ����ˮ�к��ж��ֳɷ֣�������ж������ʣ�����������ˮ�ֱ�����ͼ�������ʷ����ķ�Ӧ���(a��b��c��d���غϲ��ִ������ʼ䷴Ӧ������ˮ����)��
��.(1)��֤����ˮ����Ư���Ե���________(����a������b������c������d��)��
(2)c�����������__________________________________________________��
e�����������_________________________________________________��
e���̵ķ�Ӧ����ʽ____________________________________________��
b�����еĻ�ѧ����ʽΪ______________________________________________��
(3)���õ���ˮ��Ϊ______���û�ѧ��Ӧ����ʽ��ʾΪ_____________________��
(4)ʵ���ұ��汥����ˮ�ķ�����_______________________________��
��.�õιܽ����Ƶı�����ˮ�������뺬��̪��NaOHϡ��Һ�С����ε����һ��ʱ��ɫͻȻ��ȥ���Է�����ɫ��ԭ��
(1)������__________________________________________��
(2)������___________________________________________________��
����������ʵ��֤����ɫ��ȥ��ԭ����(1)����(2)��_______________��
���𰸡�d �а�ɫ�������� ��ˮ��dz����ɫ��ʧ Cl2��2NaOH===NaCl��NaClO��H2O Na2CO3��2HCl===2NaCl��H2O��CO2�� ϡ���� 2HClO![]() 2HCl��O2�� ��������������ɫ�Լ�ƿ���ܷⱣ�� ��ˮ��NaOH��Ӧ�����Լ��� ��ˮ�е�HClO��Ư���ԣ�����̪Ư�� ����ɫ�����Һ���ټ��������NaOH��Һ������죬˵��ԭ��(1)��ȷ������ԭ��(2)��ȷ
2HCl��O2�� ��������������ɫ�Լ�ƿ���ܷⱣ�� ��ˮ��NaOH��Ӧ�����Լ��� ��ˮ�е�HClO��Ư���ԣ�����̪Ư�� ����ɫ�����Һ���ټ��������NaOH��Һ������죬˵��ԭ��(1)��ȷ������ԭ��(2)��ȷ
��������
��ˮ�д������ַ��ӣ�Cl2��H2O��HClO���������ӣ�Cl����H����OH����ClO����������ʵ����ʷ������
��.(1)HClO����Ư���ԣ�����ˮ��ʹʯ���ȱ�����ɫ������֤����ˮ����Ư���Ե���ѡ��d��
(2)��ˮ�к��������ӣ����������ӽ�������Ȼ�����ɫ��������c�����е��������а�ɫ�������ɣ���ˮ��������������Һ��Ӧ������ˮ��dz����ɫ����e�����е���������ˮ��dz����ɫ��ʧ����Ӧ�ķ�Ӧ����ʽΪCl2��2NaOH��NaCl��NaClO��H2O����������̼���Ʒ�Ӧ����b�����еĻ�ѧ����ʽΪNa2CO3��2HCl��2NaCl��H2O��CO2����
(3)�������ֽ������Ȼ������������˾��õ���ˮ��Ϊϡ���ᣬ�û�ѧ��Ӧ����ʽ��ʾΪ2HClO![]() 2HCl��O2����
2HCl��O2����
(4)����������ֽ������Ȼ�������������ʵ���ұ��汥����ˮ�ķ�������������������ɫ�Լ�ƿ���ܷⱣ�档
��.�õιܽ����Ƶı�����ˮ�������뺬��̪��NaOHϡ��Һ�С����ε����һ��ʱ��ɫͻȻ��ȥ����ɫ��ԭ�������������ˮ��NaOH��Ӧ�����Լ�����������������ˮ�е�HClO��Ư���ԣ�����̪Ư�ף���������ڴ������Ư��ʹ����ɫ��������ɫ�����Һ�м�����������������Һ������ֺ�ɫ�������ʵ��֤����ɫ��ȥ��ԭ����(1)����(2)�ķ���Ϊ����ɫ�����Һ���ټ��������NaOH��Һ������죬˵��ԭ��(1)��ȷ������ԭ��(2)��ȷ��

����Ŀ�����������������еķ�ӦΪ2NO2(g)��O3(g)![]() N2O5(g)��O2(g)�����˷�Ӧ�ں����ܱ������н��У�����ѡ�����й�ͼ���Ӧ�ķ�����ȷ����( )
N2O5(g)��O2(g)�����˷�Ӧ�ں����ܱ������н��У�����ѡ�����й�ͼ���Ӧ�ķ�����ȷ����( )
A | B | C | D |
|
|
|
|
ƽ������£�NO2�������� | 0��2 s�ڣ�v(O3)��0.2 mol��L��1��s��1 | v����b��>a�� b�㣺v��>v�� | ���£�t1ʱ�ٳ���O3 |
A. AB. BC. CD. D