��Ŀ����
��25mL0.1mol?L-1��NaOH��Һ����μ���0.2mol?L-1��CH3COOH��Һ����ҺpH�仯������ͼ1��ʾ��
��1��B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��Һ��CH3COOH��Һǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ��______����ǡ������������ȷ�������ǡ����ȫ��Ӧ�ĵ�����______���AB������BC����CD���������ڣ�
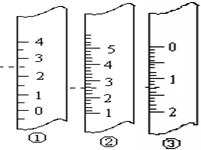
��2�����ڸõζ�ʵ�飬������ѡ����ѡ����ǡ����һ�ѡ�õζ�����ͼ2��ʾ��______��
��3����D��ʱ����Һ��c��CH3COO-��+c��CH3COOH��______2c��Na+�������������������=������
��4����C�㣬��Һ������Ũ���ɴ�С��˳��Ϊ��______��

��1��B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��Һ��CH3COOH��Һǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ��______����ǡ������������ȷ�������ǡ����ȫ��Ӧ�ĵ�����______���AB������BC����CD���������ڣ�
��2�����ڸõζ�ʵ�飬������ѡ����ѡ����ǡ����һ�ѡ�õζ�����ͼ2��ʾ��______��
| ��ƿ�е���Һ | �ζ����е���Һ | ѡ��ָʾ�� | ѡ�õζ��� | |
| A | �� | �� | ʯ�� | �� |
| B | �� | �� | ���� | �� |
| C | �� | �� | ��̪ | �� |
| D | �� | �� | ��̪ | �� |
��4����C�㣬��Һ������Ũ���ɴ�С��˳��Ϊ��______��

��1��NaOH��Һ��CH3COOH��Һǡ����ȫ��Ӧʱ�������˴����ƣ����������ˮ�⣬��Һ��ʾ���ԣ���Һ��pH����7������ǡ����ȫ��Ӧʱ��Һ��pH����7������Ӧ����AB�Σ�
�ʴ�Ϊ����AB��
��2�����������Ϣ����ƿ��Ӧ��ʢ������������Һ���ζ�����ʢ�Ŵ��ᣬ����ǡ�÷�Ӧʱ��Һ��ʾ���ԣ�Ӧ��ʹ�÷�̪��Ϊָʾ��������C��ȷ��
��ѡC��
��3��D��ʱ��������Ϊ25mL��n��CH3COO-��+n��CH3COOH��=0.2mol?L-1��0.025L=0.005mol��
n��Na+��=0.1mol?L-1��0.025L=0.0025mol������n��CH3COO-��+n��CH3COOH��=2n��Na+����
������Һ�������ͬ������c��CH3COO-��+c��CH3COOH��=2c��Na+����
�ʴ�Ϊ��=��
��4����C�㣬��Һ��pH��7��������Ũ�ȴ�������������Ũ�ȣ����ݵ���غ㣺CH3COO-+OH-=Na++H+��֪����Һ�У�c��CH3COO-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ����AB��
��2�����������Ϣ����ƿ��Ӧ��ʢ������������Һ���ζ�����ʢ�Ŵ��ᣬ����ǡ�÷�Ӧʱ��Һ��ʾ���ԣ�Ӧ��ʹ�÷�̪��Ϊָʾ��������C��ȷ��
��ѡC��
��3��D��ʱ��������Ϊ25mL��n��CH3COO-��+n��CH3COOH��=0.2mol?L-1��0.025L=0.005mol��
n��Na+��=0.1mol?L-1��0.025L=0.0025mol������n��CH3COO-��+n��CH3COOH��=2n��Na+����
������Һ�������ͬ������c��CH3COO-��+c��CH3COOH��=2c��Na+����
�ʴ�Ϊ��=��
��4����C�㣬��Һ��pH��7��������Ũ�ȴ�������������Ũ�ȣ����ݵ���غ㣺CH3COO-+OH-=Na++H+��֪����Һ�У�c��CH3COO-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����

��ϰ��ϵ�д�
�����Ŀ