��Ŀ����
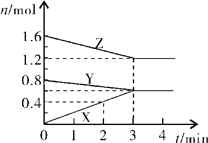
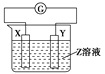
����Ŀ��40��ʱ���ڰ���ˮ��ϵ�в���ͨ��CO2���������ӵı仯��������ͼ��ʾ������˵������ȷ���ǣ� ��
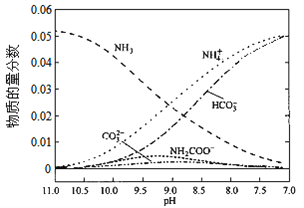
A. ��pH��9.0ʱ��c(NH4+����c(HCO3������c(NH2COO������c(CO32����
B. ��ͬpH����Һ�д��ڹ�ϵ��c(NH4+)��c(H+����2c(CO32������c(HCO3������c(NH2COO������c(OH��)
C. ����Һ��pH���Ͻ��͵Ĺ����У��к�NH2COO�����м��������
D. ����CO2��ͨ�룬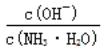 ��������
��������
���𰸡�D
����������ͼ����pH=9�ĵ㣬��һ�����߾Ϳ��Դ����ж���Ũ�ȣ�������c(NH4+����c(HCO3������c(NH2COO������c(CO32������ѡ��A��ȷ��c(NH4+)��c(H+����2c(CO32������c(HCO3������c(NH2COO������c(OH��)����Һ�ĵ���غ����������κ�pH�¶���������ѡ��B��ȷ������pH�Ľ��ͣ�NH2COO����Ũ������˵�������˺���NH2COO���Ļ��������NH2COO����Ũ���ּ�С��˵���û������ֱ���Ӧ��˵������NH2COO���Ļ��������м���ѡ��C��ȷ������һˮ�ϰ��ĵ���ƽ�ⳣ��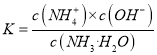 ���õ�
���õ�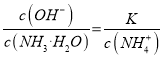 ����Ϊͨ�������̼ʵ�ʾ��Ǽ���̼�ᣬ������c(NH4+)һ������������
����Ϊͨ�������̼ʵ�ʾ��Ǽ���̼�ᣬ������c(NH4+)һ������������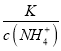 ��С����
��С����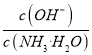 ��С��ѡ��D����
��С��ѡ��D����
�����͡���ѡ��
��������
17
����Ŀ�����������У�A��KI��s�� B��C2H5OH��l�� C��Cl2��g�� D��CH3COOH��l��E��BaSO4��s�� F��NaHSO4��aq����G��ʯī��s����H�����ǣ�s����I��NaOH��l�� J.���� K.����
��1�����ڷǵ���ʵ���____________������ţ���ͬ��������ǿ����ʵ���____________������������ʵ���____________��
��2����ֱ�ӵ������________________���������������ˮ,��ˮ��Һ�ܵ�����________________��
���𰸡� BH AEI J D FGIK ADIJ
������������������������κͻ��ý������������ǵ�����dz��˵����֮������л�������ǿ�������ǿ����ǿ���������������������������ˮ��ע�⣺���ʼȲ��ǵ����Ҳ���Ƿǵ���ʡ���1�����ڷǵ���ʵ����Ҵ������ǣ�ǿ������ǵ⻯�أ��Σ������ᱵ���������ƺ����ᣨע�⣺F������������Һ��K��HCl��Һ�������ڻ�������������Ǵ��ᡣ
��2�����嵼��Ҫ���ǽ�������ʯī������״̬�����Ҫ�������ӻ������Һ����Ҫ������Һ�д�����ֻ���ƶ������ӣ�����ʵ��룩������ֱ�ӵ����������������Һ��ʯī������������Һ�����ᡣ���ڵ���ʣ�������ˮ�����ܵ��磨���������࣬�����������ˮ��������Һһ�����磩���ǵ⻯�ء����ᡢ�������ơ����ᡣ���ᱵ���ܡ�

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�����Ŀ��X��Y��������������Z��Һ�й�����ͼ��ʾ��װ�ã�ʵ���е�����ָ�뷢��ƫת��ͬʱX����֣�Y����ϸ����X��Y��Z��Һ�����������е�(����)
��� | X | Y | Z��Һ |
|
A | Zn | Cu | ϡ���� | |
B | Cu | Zn | ϡ���� | |
C | Cu | Ag | ����ͭ��Һ | |
D | Ag | Zn | ��������Һ |
A. A B. B C. C D. D