��Ŀ����
����Ŀ���ں������������������£���Һ��NH4+��ת��ΪN2(g)��H2O(1)��ʾ��ͼ���£�
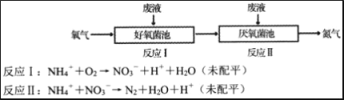
����˵����ȷ����
A. ���ط����ķ�Ӧ�У���Ԫ��ֻ������
B. ���³�ѹ�£���ӦII������8.96 L N2ʱ��ת�Ƶ�����Ϊ1.5NA
C. ������������������Ͷ���Һ�����֮��Ϊ3��5ʱ��NH4+����ȫת��ΪN2
D. ��Ӧ�����������뻹ԭ�����ʵ���֮��Ϊ5��3
���𰸡�C
��������
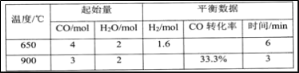
���ݷ�ӦI��NH4++2O2=NO3-+2H++H2O�ͷ�ӦII: 5NH4++3NO3-=4N2+9H2O+2H+�����������
A. I��NԪ�ش�-3������+5�ۣ���Ԫ��ֻ��������II�в���NԪ�ش�-3������0�ۣ����ִ�+5�۽���0�ۣ���Ԫ�ؼȱ������ֱ���ԭ����A����
B. ���³�ѹ�£�8.96 L N2��������N2�����ʵ�������B����
C.���ݷ�ӦI��NH4++2O2=NO3-+2H++H2O��3mol NH4+����3mol NO3-���ٸ��ݷ�ӦII: 5NH4++3NO3-=4N2+9H2O+2H+��3mol NO3-��������5mol NH4+���ʺ�����������������Ͷ���Һ�����֮��Ϊ3��5ʱ��NH4+����ȫת��ΪN2����C��ȷ��
D. ��Ӧ������������NO3-����ԭ����NH4+�����ʵ���֮��Ϊ3��5����D����
��ѡC��

 ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д�����Ŀ��ZrO2�������մɲ��ϣ������Ӣɰ����Ҫ�ɷ�ΪZrSiO4��Ҳ�ɱ�ʾΪZrO2��SiO2����������Fe2O3��Al2O3��SiO2�����ʣ�ͨ�����·�����ȡ��

��֪����ZrO2�����ռӦ���ɿ�����ˮ��Na2ZrO3��Na2ZrO3���ᷴӦ����ZrO2+��
�����ֽ���������ʵ�������¿�ʼ��������ȫ������pH���±���
�������� | Fe3+ | Al3+ | ZrO2+ |
��ʼ����ʱpH | 1.9 | 3.3 | 6.2 |
������ȫʱpH | 3.2 | 5.2 | 8.0 |
��1������ʱZrSiO4������Ӧ�Ļ�ѧ����ʽΪ ������I�Ļ�ѧʽΪ ��
��2��Ϊʹ��ҺI���������ӳ�����ȫ�����ð�ˮ��pH=a����a�ķ�Χ�� �������Ӱ�ˮ��pH=bʱ����������Ӧ�����ӷ���ʽΪ ��
��3�������III������Һ�м���CaCO3��ĩ�����ȣ��õ��������塣�÷�Ӧ�����ӷ���ʽΪ ��
��4��Ϊ�õ�������ZrO2��Zr��OH��4��Ҫϴ�ӣ�����Zr��OH��4�Ƿ�ϴ�Ӹɾ��ķ����� ��