��Ŀ����
��״���£���1L���ܺ���N2��H2��CO��NH3��NO2��H2S���ʵ���ɫSO2���壬����ͨ������ͼ��ʾ��װ�á�(��������Һ���Ϊ����)
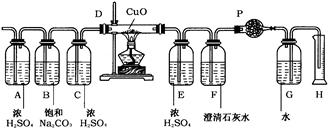
��仯����ǣ�������ͨ��Aƿ��ŨH2SO4�����Ա仯����������ޱ仯��������ͨ��Bƿ������Na2CO3��Һ��������0.5g����������ޱ仯��������ͨ��Cƿ����ͨ��D�ܣ����ȵ�CuO���к�ɫ�������ɡ���ȴ�����������Ϊ888ml(��״��)���ܽ���������ͨ��E����ͨ��F�У�����ʯ��ˮ����ǣ�ͬʱ����1.54g�������徭�������P������ͲH�У��ռ���ˮ104ml����������ʵ��ش�
��1�����������SO2���������Ϊ______________��
��2����������к��е�����Ϊ______________��
��3������������Ƿ���H2��CO���ʣ����У�ָ������������ޣ�˵�����ɡ�
a.H2��____________________________________________________________________��
b.CO�� ____________________________________________________________________��

��仯����ǣ�������ͨ��Aƿ��ŨH2SO4�����Ա仯����������ޱ仯��������ͨ��Bƿ������Na2CO3��Һ��������0.5g����������ޱ仯��������ͨ��Cƿ����ͨ��D�ܣ����ȵ�CuO���к�ɫ�������ɡ���ȴ�����������Ϊ888ml(��״��)���ܽ���������ͨ��E����ͨ��F�У�����ʯ��ˮ����ǣ�ͬʱ����1.54g�������徭�������P������ͲH�У��ռ���ˮ104ml����������ʵ��ش�
��1�����������SO2���������Ϊ______________��
��2����������к��е�����Ϊ______________��
��3������������Ƿ���H2��CO���ʣ����У�ָ������������ޣ�˵�����ɡ�
a.H2��____________________________________________________________________��
b.CO�� ____________________________________________________________________��
��1��56%����2��N2��H2��CO����3��H2��112mL����4��CO��224mL
������ͨ��ŨH2SO4������ޱ仯��˵����NH3��H2S��H2S��ŨH2SO4����������ԭ��Ӧ����
������ͨ������Na2CO3��Һ��������0.5g�������˷�Ӧ��
SO2+Na2CO3��Na2SO3+CO2��������m
22.4L�������������44g������(64-44)g
V(SO2)������ ��� m(CO2)����0.5g
V(SO2)��0.56L m(CO2)��1.1g
��(SO2)��0.56L/1L��100%��56%
��ͨ�����ȵ�CuO�к�ɫ�������ɣ�˵���л�ԭ������H2��CO�����߽��С�
��ͨ�����ʯ��ˮ����ǣ�˵����CO2(�����������)����Ϊ1.54g������еĻ�ԭ������غ�CO��
CO ����������CO2 �� Ca(OH)2
22.4L����������4g
V(CO)��������(1.54g-1.1g)
V(CO)��22.4L��0.44g/44g��0.224L��224mL
�����õ�������ֻ����N2����V(N2)��V(H2O)��104mL��S��V(H2)��1000ml-888mL��112mL��
������ͨ������Na2CO3��Һ��������0.5g�������˷�Ӧ��
SO2+Na2CO3��Na2SO3+CO2��������m
22.4L�������������44g������(64-44)g
V(SO2)������ ��� m(CO2)����0.5g
V(SO2)��0.56L m(CO2)��1.1g
��(SO2)��0.56L/1L��100%��56%
��ͨ�����ȵ�CuO�к�ɫ�������ɣ�˵���л�ԭ������H2��CO�����߽��С�
��ͨ�����ʯ��ˮ����ǣ�˵����CO2(�����������)����Ϊ1.54g������еĻ�ԭ������غ�CO��
CO ����������CO2 �� Ca(OH)2
22.4L����������4g
V(CO)��������(1.54g-1.1g)
V(CO)��22.4L��0.44g/44g��0.224L��224mL
�����õ�������ֻ����N2����V(N2)��V(H2O)��104mL��S��V(H2)��1000ml-888mL��112mL��

��ϰ��ϵ�д�
 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�
�����Ŀ
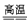 BaS+4CO��
BaS+4CO��