��Ŀ����
����Ŀ����1�������о����У���NaOH����Na2S����(NH4)2S����Na2O2����C2H2����SiC�����мȺ������Ӽ��ֺ��зǼ��Թ��ۼ������Ӿ�����__________�����мȺ������Ӽ����ֺ��м��Թ��ۼ�����λ�������Ӿ�����___________�����к��м��Թ��ۼ��ͷǼ��Թ��ۼ��ķǼ��Է�����____________����������ԭ�Ӿ������____________��
��2��Feԭ�ӻ�������Χ�н϶���������Ŀչ��������һЩ���ӻ������γ�����
�� ��Feԭ�ӻ������γ������ķ��ӻ����Ӷ��߱��Ľṹ�ص���_______________��
�� ������������ӡ�Fe(CN)6��4-�е�����CN-��Cԭ�ӵ��ӻ����������________��д��һ����CN��Ϊ�ȵ�����ĵ��ʷ��ӵĽṹʽ_______��
��3�����Ȼ���������Ϊ���壬�۵�282�棬�е�315�棬��300��������������������ˮ��Ҳ���������ѣ���ͪ���л��ܼ����ݴ��ж����Ȼ�����������Ϊ_________________��
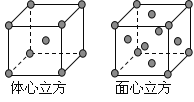
��4���������ľ����ڲ�ͬ�¶��������ֶѻ���ʽ�������ֱ�����ͼ��ʾ������������������������������ʵ�ʺ��е�Feԭ�Ӹ���֮��Ϊ_______________________��
���𰸡� �� �� �� �� ���й¶Ե��� sp N��N ���Ӿ��� 1:2
����������������1�����Ӽ�ͨ�����Ӽ��γɵľ��������Ӿ��壬���Ӽ�ͨ�����Ӽ��������γɵľ����Ƿ��Ӿ��壬ԭ�Ӽ�ͨ�����ۼ��γɵĿռ���״�ṹ�ľ�����ԭ�Ӿ��壬������Ӽ����ۼ����γɷ������
��2���ٺ��пչ���ͺ��йµ��ӶԵ�ԭ��֮�����γ���λ����
��CN����Cԭ�Ӽ۲���ӶԸ�����2���Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������ж�Cԭ���ӻ����ͣ�ԭ�Ӹ�����ȡ��۵�������ȵ�����Ϊ�ȵ����壻
��3�����Ӿ����۷е�ϵͣ�
��4�����þ�̯�ּ���ÿ��������ԭ�Ӹ�����
��⣺��1����NaOH�к������Ӽ��ͼ��Լ�����Na2S��ֻ�����Ӽ�����(NH4)2S�к������Ӽ��ͼ��Լ�����Na2O2�к������Ӽ��ͷǼ��Լ�����C2H2�к��м��Լ��ͷǼ��Լ�����SiC��ֻ�м��Լ��������мȺ������Ӽ��ֺ��зǼ��Թ��ۼ������Ӿ����ǹ������ƣ����мȺ������Ӽ����ֺ��м��Թ��ۼ�����λ�������Ӿ�����(NH4)2S����Ȳ��ֱ���νṹ��������к��м��Թ��ۼ��ͷǼ��Թ��ۼ��ķǼ��Է�������Ȳ����������ԭ�Ӿ������SiC��
��2���ٺ��пչ���ͺ��йµ��ӶԵ�ԭ��֮�����γ���λ����Feԭ�Ӻ��пչ������Feԭ���ܺͺ��йµ��ӶԵ�ԭ�����γ���λ����
��CN-��Cԭ�Ӽ۲���ӶԸ�����2���Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������ж�Cԭ���ӻ�����Ϊsp��ԭ�Ӹ�����ȡ��۵������ֱ���ȵ�����Ϊ�ȵ����壬����������к���2��ԭ�ӡ��۵�������10���뵪�����ӻ�Ϊ�ȵ����壬�������Ӻ�����������ṹʽΪN��N��
��3�����Ӿ����۷е�ϵͣ����������Ϣ֪���Ȼ����۷е�ϵͣ�Ӧ���Ƿ��Ӿ�����
��4��������������������Feԭ�Ӹ���=8��1/8+6��1/2=4����������������Feԭ�Ӹ���=1+8��1/8=2��������������������Feԭ�Ӹ�������������������Feԭ�Ӹ���֮��=2��4=1��2��

 53���ò�ϵ�д�
53���ò�ϵ�д�����Ŀ����Ҫ����գ�
(1)��֪R2�����ӵĺ�����n�����ӣ�R��������ΪM����mg R2�������ﺬ�е��ӵ����ʵ���Ϊ ____________mol��
(2)XԪ������������Ӧ��ˮ����ΪH3XO4��������Ӧ����̬�⻯��Ϊ_____ ��
(3)��֪ͬ����X��Y��Z����Ԫ�ص�����������Ӧˮ����������ǿ������˳��ΪHXO4��H2YO4��H3ZO4����Ԫ�طǽ�������ǿ����˳��Ϊ��____________ ����̬�⻯����ȶ�����ǿ����˳��Ϊ��______________ ��
(4).�ס������ַ�Ԫ�أ��ټױ���������H2���ϣ��ڼ�ԭ�������ҵ������ӷ����û���Ӧ���ۼ�����������Ӧ��ˮ�������Ա��ҵ�����������Ӧ��ˮ��������ǿ������ij������Ӧʱ����ԭ�ӵõ�����Ŀ���ҵĶࣻ�ݼĵ����ۡ��е���ҵĵ͡�
��˵���ķǽ����Ա��ҵķǽ�����ǿ����________________
(5)�±���ij��ȤС��ͨ��ʵ���õ���ͬ�������ϡ����������Ӧ��ʵ�����ݣ�
ʵ����� | ��������/g | ����״̬ | c(H2SO4) mol/L | ʵ���¶�/�� | ������ʧ��ʱ��/s |
1 | 0.10 | ˿ | 0.7 | 25 | 240 |
2 | 0.10 | ˿ | 1.0 | 25 | 190 |
3 | 0.10 | ��ĩ | 1.0 | 25 | 120 |
4 | 0.10 | ��ĩ | 1.0 | 40 | 40 |
�����������ݣ��ش��������⣺
��ʵ��1��
��ʵ��2��3�ɵó��Ľ�����_________________________��
��ʵ��3��4�ɵó��Ľ�����______________________��
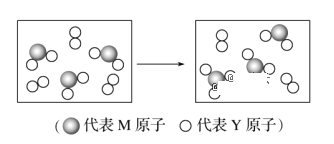
(6)��ͼ��ʾ��M��Y��Ԫ����ɵ��������������һ�������µ��ܱ������г�ַ�Ӧǰ���ת����ϵ����д����ת�����̵Ļ�ѧ����ʽ��______________________��