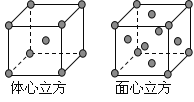
��Ŀ����
����Ŀ����ˮ��Դ�����þ��зdz�������ǰ�����Ӻ�ˮ�п���ȡ���ֻ���ԭ�ϡ�
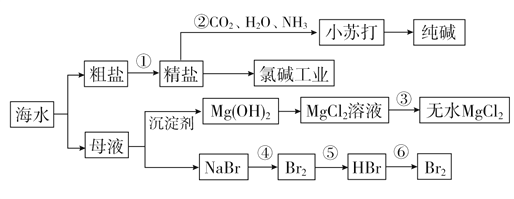
(1)��ˮ���������ķ�����__________________________(д�����ּ���)��
(2)�����к���Ca2����Mg2����SO![]() �����ʣ�����ʱ�����Լ�Ϊ��
�����ʣ�����ʱ�����Լ�Ϊ��
A������ B��BaCl2��Һ C��NaOH��Һ D��Na2CO3��Һ
�����Լ���˳����__________________��
(3)������У�������Һ��Ӧ��ͨ��________����ͨ��________��
(4)�ȼҵ�У����Դ���������ĵ缫������ҺpHֵ________(������С�����䡱)���ò�����պŨ��ˮ�����������������壬���ֲ����������̣����̵���Ҫ�ɷ���________________________��
(5)ʵ�������У���ѡ��________��Ϊ�����������Ȼ�þ��Һ�еõ���ˮ����IJ���Ϊ_____________________________________��
(6)�������SO2ˮ��Һ�����嵥�ʣ������ʿɴ�93%����Ӧ�����ӷ���ʽΪ______________________________________��
���𰸡� �����䶳�������ӽ�������Ĥ��������������������(д�����ּ���) CBDA(��BCDA��BDCA) NH3 CO2 ��� NH4Cl ʯ���� ��MgCl2��Һ��HCl���������� Br2��SO2��2H2O===4H����2Br����SO![]()
����������������1����ˮ���������ķ����������䶳�������ӽ������ȣ�
��2������Ca2����̼���Ƴ�ȥ��Mg2�����������Ƴ�ȥ��SO42�����Ȼ�����ȥ�������
��3�����ݰ�����������ˮ��������̼��ˮ�е��ܽ��С������
��4�����ݵ�ⱥ��ʳ��ˮʱ���������ӷŵ��жϣ�����������������������
��5����������������Դ�㣬�۸���˷���������þ����ˮ�������
��6��������ˮ�ܰѶ���������������������廯����
��⣺��1����ˮ���������ķ�����Ҫ�������䶳�������ӽ�������Ĥ������������������������
��2��Ca2����̼���Ƴ�ȥ��Mg2�����������Ƴ�ȥ��SO42�����Ȼ�����ȥ�������ˣ�����Һ�м��������ữ�������ڹ������Ȼ���Ҫ��̼��������������̼���Ʊ�������Ȼ����ĺ��棬���������ƿ���������������Լ����Լ���˳����CBDA(��BCDA��BDCA)��
��3�����ڰ�����������ˮ����������̼��ˮ�е��ܽ��Ժ�С������Ҫ�Ʊ�̼�����ƣ��������Ӧ��������Һ��Ӧ��ͨ�백������ͨ�������̼��
��4���ȼҵ�У����Դ���������ĵ缫����������Һ�е������ӷŵ�����������Ӷ��ƻ�������Χˮ�ĵ���ƽ�⣬������Ũ��������������������ҺpHֵ������������ӷŵ�ų���������������ǿ�����ԣ��ò�����պŨ��ˮ�����������������壬�����ܰѰ����������ɵ�����ͬʱ����ԭΪ�Ȼ��⣬�Ȼ����백����Ӧ�����Ȼ�臨������������̣������̵���Ҫ�ɷ���NH4Cl��
��5����������������Դ�㷺���۸���ˣ�����ʵ�������У���ѡ��ʯ������Ϊ�������������Ȼ�þˮ������������þ���Ȼ��⣬ˮ�����ȣ���˴��Ȼ�þ��Һ�еõ���ˮ����IJ���Ϊ��MgCl2��Һ��HCl���������ɡ�
��6����ˮ���������ԣ��ܰѶ�����������Ϊ���ᣬ��˷�Ӧ�����ӷ���ʽΪBr2��SO2��2H2O��4H����2Br����SO42����

����Ŀ�����в����ܹ��ﵽʵ��Ŀ�ĵ���
|
|
|
|
A����֤һ������SO2 | B. ���ſ������ռ�NO | C. ��ȡ�����鰱�� | D����ˮ���հ��� |
A. A B. B C. C D. D