��Ŀ����
����Ŀ��������һ����Ҫ�Ļ���ԭ�Ϻ������Դ���о���������������������Ҫ���塣��ش�
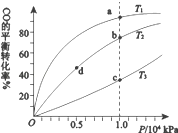
(1)��֪һ�������·������·�Ӧ��CO2(g)+2H2O(g)![]() CH4(g)+2O2(g) ��H=+802kJ��mol��1����һ������CO2(g)��H2O(g)����10L�ܱ������У��ֱ��ڴ���M��N�������·���������Ӧ��CH4(g)�IJ���(n)�����ʱ��(t)���¶�(T)�仯�Ĺ�ϵ��ͼ1��ʾ��
CH4(g)+2O2(g) ��H=+802kJ��mol��1����һ������CO2(g)��H2O(g)����10L�ܱ������У��ֱ��ڴ���M��N�������·���������Ӧ��CH4(g)�IJ���(n)�����ʱ��(t)���¶�(T)�仯�Ĺ�ϵ��ͼ1��ʾ��
���������ȼ����(��H)Ϊ��890kJ��mol��1����ˮ����������H=________��(������ָ1molҺ��ת��Ϊ����ʱ���յ�����)
��T1�桢����M�����£�0--20h�ڸ÷�Ӧ����v(H2O)=_______��

�۸���ͼ1�жϣ������Ĵ�Ч����M________N(����ǿ��������������)��
(2)����������Ʊ��ϳ�����CH4(g)+H2O(g)![]() CO(g)+3H2(g) ��H����CH4(g)��H2O(g)���ʵ���֮��Ϊ1��3����ʢ�д����ĸ��������з����÷�Ӧ����ͬʱ����ڲ��CO���������(
CO(g)+3H2(g) ��H����CH4(g)��H2O(g)���ʵ���֮��Ϊ1��3����ʢ�д����ĸ��������з����÷�Ӧ����ͬʱ����ڲ��CO���������(![]() )���¶�(T)�Ĺ�ϵ��ͼ2��ʾ��
)���¶�(T)�Ĺ�ϵ��ͼ2��ʾ��
��T0��ʱ��CO�������������ԭ��Ϊ__________��
����T0��ʱ����������ʼѹǿΪp0��CO��ƽ���������Ϊ10%����Ӧ��ƽ�ⳣ��Kp=____(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�������)��
���𰸡�![]()
![]() ���� ����T0 ��ʱ����ͬʱ������¶�Խ�߷�Ӧ����Խ�죬CO���������Խ����T0 ��ʱ����Ӧ�ﵽƽ�⣬�÷�ӦΪ���ȷ�Ӧ���¶�Խ��CO���������ԽС
���� ����T0 ��ʱ����ͬʱ������¶�Խ�߷�Ӧ����Խ�죬CO���������Խ����T0 ��ʱ����Ӧ�ﵽƽ�⣬�÷�ӦΪ���ȷ�Ӧ���¶�Խ��CO���������ԽС ![]()
��������
��1������֪�� ![]() ��
��
�����ȼ����Ϊ![]() �������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ
�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ![]() �����ݸ�˹���ɣ�����+�ڣ��ɵ�
�����ݸ�˹���ɣ�����+�ڣ��ɵ�![]() ���ݴ˼������H���ɣ�
���ݴ˼������H���ɣ�
�ڸ���ͼ���֪��T1�桢����M�����£�0��20h�ڼ�������ʵ����仯Ϊ0.6mol�����v=���������ķ�Ӧ���ʣ�Ȼ���Ϸ�Ӧ�����뻯ѧ�����������ȼ��㣻�۴�����Ӱ��ƽ�⣬�����Ĵ�Ч��Խǿ���ﵽƽ���ʱ��Խ�̣�
��2����û�дﵽƽ��״̬ʱ���¶�Խ�߷�Ӧ����Խ�죬���ﵽƽ��״̬�������¶�ƽ�����������ƶ����ݴ˷�����
��CH4��g����H2O��g�������ʵ���֮��Ϊ1��3����CH4Ϊxmol��H2OΪ3xmol��ƽ��ʱ���ɵ�COΪymol����ʽ���㼴�ɡ�
��1������֪��![]() �������ȼ����Ϊ
�������ȼ����Ϊ![]() �������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ
�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ![]() �����ݸ�˹���ɣ�����+�ڣ��ɵã�
�����ݸ�˹���ɣ�����+�ڣ��ɵã�![]() ��
��
�ʴ�Ϊ��![]() ��
��
�ڸ���ͼ���֪��T1�桢����M�����£�0��20h��ˮ�����ʵ����仯Ϊ0.6mol����![]() ������
������![]() ��
��
�ʴ�Ϊ��![]() ��
��
�۴�����Ӱ��ƽ�⣬�����Ĵ�Ч��Խǿ���ﵽƽ���ʱ��Խ�̣���ͼ���֪M<N,�ʴ�Ϊ�����ڣ�
��2�������¶ȵ���T0��ʱ����ͬʱ������¶�Խ�߷�Ӧ����Խ�죬��CO���������Խ�����¶�ΪT0��ʱ����Ӧ�ﵽƽ�⣬���ڸ÷�ӦΪ���ȷ�Ӧ���¶�Խ��CO���������ԽС������T0��ʱCO������������
�ʴ�Ϊ������T0��ʱ����ͬʱ������¶�Խ�߷�Ӧ����Խ�죬CO���������Խ����T0��ʱ����Ӧ�ﵽƽ�⣬�÷�ӦΪ���ȷ�Ӧ���¶�Խ��CO���������ԽС��
��![]() ��
��![]() �����ʵ���֮��Ϊ1��3����
�����ʵ���֮��Ϊ1��3����![]() Ϊxmol��
Ϊxmol��![]() Ϊ3xmol��ƽ��ʱ���ɵ�COΪymol��CO��ƽ���������Ϊ10%
Ϊ3xmol��ƽ��ʱ���ɵ�COΪymol��CO��ƽ���������Ϊ10%
![]()
��ʼ��mol�� x 3x 0 0
ת�� y y y 3y
ƽ�� x-y 3x-y y 3y
��![]() ����ã�y=0.5x��ƽ��ʱ�������������ʵ���Ϊ��
����ã�y=0.5x��ƽ��ʱ�������������ʵ���Ϊ��![]() ����Ӧǰ�����ʵ���Ϊ4xmol��T0��ʱ����������ʼѹǿΪp0����ƽ��ʱѹǿΪ��
����Ӧǰ�����ʵ���Ϊ4xmol��T0��ʱ����������ʼѹǿΪp0����ƽ��ʱѹǿΪ��![]()
![]()
![]() ��
��![]() ��
��![]() ����Ӧ��ƽ�ⳣ��Kb=
����Ӧ��ƽ�ⳣ��Kb=![]() =
=![]() ��
��
�ʴ�Ϊ��![]() ��
��

����Ŀ������������ѧ��Ӧ��ƽ�ⳣ����K1��K2��K3�����¶ȵĹ�ϵ�ֱ����±���ʾ��
��ѧ��Ӧ | ƽ�ⳣ�� | �¶� | |
973K | 1173K | ||
��Fe��s��+CO2��g�� | K1 | 1.47 | 2.15 |
��Fe��s��+H2O��g�� | K2 | 2.38 | 1.67 |
��CO��g��+H2O��g�� | K3 | �� | �� |
������˵����ȷ����
A����H1��0����H2��0
B����Ӧ�٢ڢ��ķ�Ӧ�������ϵ����H2����H1����H3
C����Ӧ�٢ڢ���ƽ�ⳣ�������ϵ��K1��K2��K3
D��Ҫʹ��Ӧ����һ�������½�����ƽ��������Ӧ�����ƶ����ɲ�ȡ���´�ʩ
����Ŀ����һ�����İ�������粒�������ij�ݻ��㶨����������У�������Ӧ��H2NCOONH4(s)![]() 2NH3(g)��CO2(g)���ڲ�ͬ�¶��£��÷�Ӧ��ƽ��״̬ʱ�IJ������������ʾ������˵����ȷ���ǣ� ��
2NH3(g)��CO2(g)���ڲ�ͬ�¶��£��÷�Ӧ��ƽ��״̬ʱ�IJ������������ʾ������˵����ȷ���ǣ� ��
�¶� | ƽ��Ũ��(mol��L��1) | |
c(NH3) | c(CO2) | |
T1 | 0.1 | |
T2 | 0.1 | |
A.��T2��T1����÷�Ӧ����H��0
B.���������N2��H2NCOONH4��������
C.NH3�����������ʱ��˵���÷�Ӧ�ﵽƽ��
D.T1��T2ʱ��ת����H2NCOONH4�����ʵ�����n(T2)��2��n(T1)