��Ŀ����
����Ŀ��I������������ת����A��һ�����Σ�D����Է���������C����Է���������16��E���ᣬ��X������ǿ�ỹ��ǿ��ʱ���������µ�ת����ϵ��
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
��X��ǿ��ʱ��A��B��C��D��E����ͬһ��Ԫ�أ���X��ǿ��ʱ��A��B��C��D��E���������ͬһ��Ԫ�ء���ش�
��1��A�Ļ�ѧʽ��______�� Z�ĵ���ʽ��______��
��2����X��ǿ��ʱ��д��C��D�Ļ�ѧ����ʽ��_____��
��3����X��ǿ��ʱ��д��E��ϡ��Һ��ͭ��Ӧ����C�����ӷ���ʽ��_____��
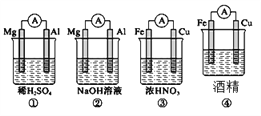
II����FeSO4��Һ�м���(NH4)2SO4������Ʊ�Ħ���ξ���[(NH4)2SO4��FeSO4��6H2O] (����Է�������Ϊ392)���þ����һ���������ȶ������ױ�������������ˮ���������Ҵ���
��4��Ϊ��ϴ��(NH4)2SO4��FeSO4��6H2O�ֲ�Ʒ�����з���������ʵ���____(����ĸ����)��
A������ˮϴ B��������ˮϴ��������ˮ�Ҵ�ϴ
C����30%���Ҵ���Һϴ D����90%���Ҵ���Һϴ
��5��Ϊ�˲ⶨ��Ʒ�Ĵ��ȣ���ȡa g��Ʒ����ˮ�����Ƴ�500mL��Һ����Ũ��Ϊc mol��L-1������KMnO4��Һ�ζ���ÿ����ȡ����Һ�����Ϊ25.00mL��ʵ������¼���£�
ʵ����� | ��һ�� | �ڶ��� | ������ |
���ĸ��������Һ���/mL | 25.52 | 25.02 | 24.98 |
���ϱ��е�һ��ʵ���м�¼�������Դ��ں����Σ���ԭ�������_____(����ĸ����)��
A���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
B����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ
C�������Ը�����ر�Һ����ʱ��������в��ֱ��ʣ�Ũ�Ƚ���
D��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���Ը��������Һ�����
��ͨ��ʵ�����ݼ���ĸò�Ʒ����Ϊ_____(����ĸa��c��ʾ)��
���𰸡� (NH4)2S ![]() 2SO2��O2
2SO2��O2 ![]() 2SO3 3Cu��8H+ ��2NO3�� === 3Cu2+��2NO����4H2O D B
2SO3 3Cu��8H+ ��2NO3�� === 3Cu2+��2NO����4H2O D B ![]() ��100%
��100%
�����������������I�� D����Է���������C�Ĵ�16������һ�������������������ͨ�������ɳ����ж�D��C��һ����ԭ�ӣ���������ת����ϵ���У�SO2��SO3��NO��NO2��Na2SO3��Na2SO4�ȣ��ɴ˿ɳ��ƶ�YΪO2������EΪ�ᣬ��DӦΪ��ת��Ϊ���ij���ʣ��ܿ���ΪSO3��NO2�ȣ���DΪSO3��˳��EΪH2SO4��ZΪH2O������BΪH2S��AΪ�����ʱXΪǿ���DΪNO2��˳��EΪHNO3��ZΪH2O������BΪNH3��AΪ��Σ��ۺ϶���AӦΪ��NH4��2S���Դ˽����⡣
II����4������(NH4)2SO4��FeSO4��6H2O���ܽ���ѡ��ϴ�Ӽ�����5��������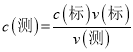 ���� ���ڵ�һ��ʵ���м�¼�������Դ��ں���������һ��ʵ��¼������ȥ���õڶ��Ρ�������ʵ�����ݼ������ĸ��������Һ��������ù�ϵʽ5(NH4)2SO4��FeSO4��6H2O
���� ���ڵ�һ��ʵ���м�¼�������Դ��ں���������һ��ʵ��¼������ȥ���õڶ��Ρ�������ʵ�����ݼ������ĸ��������Һ��������ù�ϵʽ5(NH4)2SO4��FeSO4��6H2O![]() KMnO4 ������ĸò�Ʒ������
KMnO4 ������ĸò�Ʒ������
��������1�������Ϸ�����֪��A�ǣ�NH4��2S��Y��O2��Z��H2O��ˮ�ĵ���ʽ��![]() ����2����X��ǿ��ʱ��E��H2SO4��C����D�Ļ�ѧ����ʽ2SO2��O2
����2����X��ǿ��ʱ��E��H2SO4��C����D�Ļ�ѧ����ʽ2SO2��O2 ![]() 2SO3��
2SO3��
��3����X��ǿ��ʱ��E��HNO3��������ͭ��Ӧ�����ӷ���ʽ��3Cu��8H+ ��2NO3�� === 3Cu2+��2NO����4H2O
II����4�� (NH4)2SO4��FeSO4��6H2O���ױ�������������ˮ���������Ҵ���Ϊ��ֹ(NH4)2SO4��FeSO4��6H2O�ܽ⣬���Ͳ��ʣ���90%���Ҵ���Һϴ��
��5��������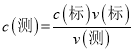 ��A���ζ�ǰ�ζ��ܼ��������ݣ��ζ����������������ĸ��������Һ���ƫС����A������B����һ�εζ��õ���ƿ�ô�װҺ��ϴ����(NH4)2SO4��FeSO4��6H2O�����ʵ���ƫ�����ĸ��������Һ���ƫ��B��ȷ��C�������Ը�����ر�Һ����ʱ��������в��ֱ��ʣ�Ũ�Ƚ��ͣ�Ӧ�ö�����ʵ�鶼��Ӱ�죬��C������D��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���Ը��������Һ��������������ĸ��������Һ���ƫС����D������
��A���ζ�ǰ�ζ��ܼ��������ݣ��ζ����������������ĸ��������Һ���ƫС����A������B����һ�εζ��õ���ƿ�ô�װҺ��ϴ����(NH4)2SO4��FeSO4��6H2O�����ʵ���ƫ�����ĸ��������Һ���ƫ��B��ȷ��C�������Ը�����ر�Һ����ʱ��������в��ֱ��ʣ�Ũ�Ƚ��ͣ�Ӧ�ö�����ʵ�鶼��Ӱ�죬��C������D��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���Ը��������Һ��������������ĸ��������Һ���ƫС����D������
�ڵ�һ��ʵ���м�¼�������Դ��ں���������һ��ʵ��¼������ȥ���õڶ��Ρ�������ʵ�����ݼ������ĸ��������Һ���Ϊ![]() =25.00mL ����ÿ����ȡ����Һ�к�����n mol��
=25.00mL ����ÿ����ȡ����Һ�к�����n mol��
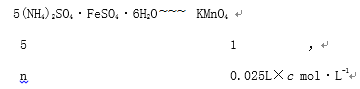
n=0.025L��c mol��L-1��5
��ò�Ʒ����Ϊ0.025L��c mol��L-1��5��20��392g/mol��ag��100%=![]() ��100%��
��100%��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����2 L�ĺ����ܱ������г���A(g)��B(g)��������Ӧ��A(g)��B(g) ![]() 2C(g)��D(s)����H��a kJ��mol��1ʵ�����ݺͽ���ֱ������ͼ��ʾ������˵����ȷ����(����)
2C(g)��D(s)����H��a kJ��mol��1ʵ�����ݺͽ���ֱ������ͼ��ʾ������˵����ȷ����(����)
ʵ�� ��� | �¶� | ��ʼ���ʵ��� | ���� �仯 | |
A | B | |||
�� | 600�� | 1 mol | 3 mol | 96 kJ |
�� | 800�� | 1.5 mol | 0.5 mol | ____ |
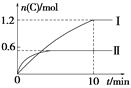
A. ʵ�����У�10 min��ƽ������v(B)��0.06 mol��L��1��min��1
B. ��������ʽ��a��160
C. 600��ʱ���÷�Ӧ��ƽ�ⳣ����0.45
D. ��ʵ������ƽ����ϵ���ٳ���0.5 mol A��1.5 mol B��A��ת��������