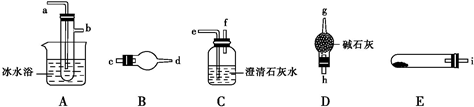
��Ŀ����
15����ͭ���ʼ��仯�����Ӧ�÷�Χ�ܹ㣮���к��Ȼ��������ʵ��Ȼ�ͭ���壬Ϊ��ȡ������CuCl2•2H2O�����Ƚ����Ƴ�ˮ��Һ��Ȼ������ͼ��������ᴿ��
��֪Cu2+��Fe3+��Fe2+���������↑ʼ�����ͳ�����ȫʱ��pH���±���
| Fe3+ | Fe2+ | Cu2+ | |
| ��ʼ������pH | 1.9 | 7.0 | 4.7 |
| ��ȫ������pH | 3.2 | a | 6.7 |
��1����ѧ��ͨ����Ϊ��������Һ�е�����Ũ��С��1��10-5mol/Lʱ�������ʹﵽ��ȫ����֪Fe��OH��2��KspԼΪ1.0��10-15����a=9��
��2��������������Ŀ���ǽ�Fe2+������Fe3+��XӦѡ��C��
A��K2Cr2O7 B��ŨHNO3 C��H2O2 D��KMnO4
��3�����������Y��CuO[Cu��OH��2��CuCO3��Cu2��OH��2CO3Ҳ����]��
��4�����ʵ�����Һ���л�ô�����CuCl2•2H2O����Ҫ����ʵ�鲽��Ӧ��HCl�����м��������ᾧ�����ˡ���ˮϴ�ӡ����º�ɣ�
���� �Ȼ��������Ȼ�ͭ�Ļ��Һ�У��������������Խ�������������Ϊ�����ӣ�����pH���Խ������ӳ������õ��Ȼ�ͭ��ˮ��Һ��Ȼ�������Ի���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����T�ɵõ��Ȼ�ͭ���壬
��1�������ܶȻ�����������2����������������Fe2+����ΪFe3+���׳�ȥ���ݴ˻ش�
��2��ѡ������������������µ����������������������
��3���ڵ���pHʱ����������ʲ������������ʣ�
��4��ͭ����ˮ����Һ��ʾ���ԣ�������������ͭ��Һ�õ�����������ͭ���ݴ˻ش�
��� �⣺��������ͼ���Ȼ��������Ȼ�ͭ�Ļ��Һ�У��������������Խ�������������Ϊ�����ӣ�����pH���Խ������ӳ������õ��Ȼ�ͭ��ˮ��Һ��Ȼ�������Ի���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����T�ɵõ��Ȼ�ͭ���壬
��1����������Һ�е�����Ũ��С��1��10-5mol/Lʱ�������ʹﵽ��ȫ����֪Fe��OH��2��KspԼΪ1.0��10-15��
Ksp=c��Fe2+��c2��OH-��=1.0��10-15
1��10-5mol/L��c2��OH-��=1.0��10-15
c��OH-��=10-5mol/L��
c��H+��=$\frac{1{0}^{-14}}{1{0}^{-5}}$=10-9mol/mol��
a=9��
�ʴ�Ϊ��9��
��2������ʵ��Ŀ�ģ�������������Ŀ���ǽ�Fe2+������Fe3+����������Fe��OH��3��������Cu2+���룬K2Cr2O7��HNO3��H2O2��KMnO4�����������ԣ��ܽ�������������������K2Cr2O7��HNO3��KMnO4�������µ��������ӣ�˫��ˮ����ɫ���������õ��Ļ�ԭ������ˮ�����������������ӣ�
�ʴ�Ϊ����Fe2+������Fe3+����������Fe��OH��3��������Cu2+���룻C��
��3���к���Һ�����ԣ�����pH���������ӳ�����ͭ���Ӳ����������Լ���CuO[Cu��OH��2��CuCO3��Cu2��OH��2CO3Ҳ����]��
�ʴ�Ϊ��CuO[Cu��OH��2��CuCO3��Cu2��OH��2CO3Ҳ����]��
��4��ͭ����ˮ����Һ��ʾ���ԣ�������������ͭ��Һ�õ�����������ͭ��Ϊ��ֹˮ�⣬����Һ���л�ô�����CuCl2•2H2O��Ӧ����HCl���������ɣ���HCl�����м���Ũ������ȴ�ᾧ�����ˡ���ˮϴ�ӡ����º�ɣ�
�ʴ�Ϊ��Ӧ��HCl�����м��������ᾧ�����ˡ���ˮϴ�ӡ����º�ɣ�
���� ������һ�����ʵķ�����ᴿ��ʵ�鷽������⣬����ѧ��������������Լ����Ӧ����ѧ֪ʶ����������Ŀ�ѶȲ���

| A�� | ��Ȳ�ͼױ� | B�� | ��ϩ�ͱ��� | C�� | �Ҵ��ͱ��� | D�� | ��ȩ�������� |
| A�� | ͭ˿�ڿ��������պ�Ѹ�������Ҵ������� | |
| B�� | ������������ʵ���֮��Ϊ1��1ʱ��ϼ��� | |
| C�� | п�ۼ��뵽�Ȼ�ͭ��Һ�� | |
| D�� | ȥ������Ĥ���������뵽Ũ������ |
 �����Ϊ2L�ĺ����ܱ������з�����ӦxA��g��+yB��g��?zC��g����ͼ�ױ�ʾ200��ʱ������A��B��C���ʵ�����ʱ��ı仯��ͼ�ұ�ʾ��ͬ�¶���ƽ��ʱC�������������ʼn��A����n��B���ı仯��ϵ�������н�����ȷ����
�����Ϊ2L�ĺ����ܱ������з�����ӦxA��g��+yB��g��?zC��g����ͼ�ױ�ʾ200��ʱ������A��B��C���ʵ�����ʱ��ı仯��ͼ�ұ�ʾ��ͬ�¶���ƽ��ʱC�������������ʼn��A����n��B���ı仯��ϵ�������н�����ȷ������������
| A�� | 200��ʱ����Ӧ�ӿ�ʼ��ƽ���ƽ������v��B��=0.04 mol•L-1•min-1 | |
| B�� | ��ͼ�ҿ�֪����ӦxA��g��+yB��g��?zC��g���ġ�H��0����a=2 | |
| C�� | ����2 L���ݾ��ȣ������û�������������ܱ��������и÷�Ӧ����ѧƽ�ⳣ����С���ﵽƽ��ʱ��A���������С��0.5 | |
| D�� | 200��ʱ����ʼ�������г���0.2 mol A��0.4 mol B��0.4 mol C����Ӧ�ﵽƽ��ǰ�����ʣ�v��������v���棩 |
| A�� | 9 g | B�� | 10 g | C�� | 11 g | D�� | 12 g |
| A�� | �ڱ�״���£�560 mL�������ȩ��ɵĻ�����к��еĹ��õ��Ӷ���Ϊ0.1 NA | |
| B�� | ��⾫��ͭʱת����6.02��1023�����ӣ������ܽ�32 gͭ | |
| C�� | 2.24 LCO��2.8gN2��ɵĻ������������Ϊ2.8 NA | |
| D�� | ��0.2 mol H2SO4��Ũ����������ͭ��Ӧ������SO2�ķ�����Ϊ0.1��6.02��1023 |
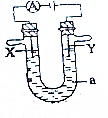 ���ԭ���ڻ�ѧ��ҵ�����Ź㷺��Ӧ�ã�ͼ�е���aΪ���Һ��X��Y������缫�壮��
���ԭ���ڻ�ѧ��ҵ�����Ź㷺��Ӧ�ã�ͼ�е���aΪ���Һ��X��Y������缫�壮�� ��͵����������γ����ꡢ�����Ȼ�����Ⱦ��������ף����ú��ʵĴ�ʩ��������Ⱦ�DZ�����������Ҫ�ٴ룮
��͵����������γ����ꡢ�����Ȼ�����Ⱦ��������ף����ú��ʵĴ�ʩ��������Ⱦ�DZ�����������Ҫ�ٴ룮