��Ŀ����
17��ijʵ��С��������װ�ý����Ҵ���������ʵ�飮
��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ����ʽ2Cu+O2$\frac{\underline{\;\;��\;\;}}{\;}$2CuO��CH3CH2OH+CuO$\stackrel{��}{��}$CH3CHO+Cu+H2O��
��2����������ˮԡ���ò���ͬ���������Ǽ��ȣ�ʹ�Ҵ��ӷ����ҵ���������ȴ���ռ����
��3�����з�Ӧԭ���������ڼ��˾���Ƿ�ƺ���
2K2Cr2O7+3C2H5OH+8H2SO4=2Cr2��SO4��3+3CH3COOH+2K2SO4+11H2O
����ɫ�� ����ɫ��
����˾���ƺ���Ӧ��ʾ����ɫ��
������1mol Cr3+ת�Ƶĵ�����Ϊ1.8��1024��3NA��
���� ��1���Ҵ��Ĵ�������Ӧʵ���ǣ�����ͭ����������Ϊ����ͭ������ͭ���Ҵ�����Ϊ��ȩ��ͭ������ͭ��������ã�
��2���Ҵ��������ӷ��������ױ�ɾ�����ʽ��
��3���ٸ��ݷ�Ӧ����ʽ��֪���Ҵ���K2Cr2O7����ɫ����Ӧ����Cr2��SO4��3����ɫ�����ݴ��жϼ��������������ɫ��
�ڷ�Ӧ���Ԫ�صĻ��ϼ�Ϊ+6�ۣ���Ӧ���Ϊ+3�ۣ����ϼ۱仯Ϊ3���ݴ˼����ת�Ƶ�������
��� �⣺��1���Ҵ��Ĵ�������Ӧ�У�����ͭ���������������Ҵ���Ӧ���������õ�������ͭ����Ӧ��ԭ���ǣ�2Cu+O2$\frac{\underline{\;\;��\;\;}}{\;}$2CuO��CH3CH2OH+CuO$\stackrel{��}{��}$CH3CHO+Cu+H2O��
�ʴ�Ϊ��2Cu+O2$\frac{\underline{\;\;��\;\;}}{\;}$2CuO��CH3CH2OH+CuO$\stackrel{��}{��}$CH3CHO+Cu+H2O��
��2����������ˮԡ���ò���ͬ��������ˮԡ�����������Ҵ�ƽ���������Ҵ�������������ˮԡ������������16.6�����Ծ�����ʽ���ڣ������ҵ������ǽ��������ȴ��
�ʴ�Ϊ�����ȣ�ʹ�Ҵ��ӷ�����ȴ���ռ����
��3���ٸ��ݷ�Ӧ2K2Cr2O7����ɫ��+3C2H5OH+8H2SO4=2Cr2��SO4��3����ɫ��+3CH3COOH+2K2SO4+11H2O��֪����˾���ƺ����Ҵ���2K2Cr2O7����ɫ������������ԭ��Ӧ����2Cr2��SO4��3����ɫ����������ʾ��ɫ��
�ʴ�Ϊ���̣�
��K2Cr2O7�и�Ԫ�صĻ��ϼ�Ϊ+6�ۣ�Cr3+�и�Ԫ�صĻ��ϼ�Ϊ+3�ۣ�������1molCr3+ת���ˣ�6-3��mol=3mol���ӣ�
ת�Ƶĵ�����Ϊ��N=3NA=1.8��1024��
�ʴ�Ϊ��1.8��1024��3NA��
���� ���⿼���Ҵ��Ļ�ѧ���ʣ���Ŀ�Ѷ��еȣ�ע�������Ҵ��Ļ�ѧ���ʣ���ȷ�Ҵ��Ĵ�������Ӧԭ��������������ѧ���ķ���������������������ѧʵ��������

| A�� | ֻ�Т� | B�� | ֻ�Т� | C�� | �٢ڢ۵Ļ���� | D�� | �٢ڢۢܵĻ���� |
| ���Ӵ��� | X | Y | Z | W |
| ԭ�Ӻ��� | ���� | ��ͬԪ�ع��ɵ����� | ͬԪ�ع��ɵ����� | ͬԪ�ع��ɵ����� |
| ���ӵĵ���� | 0 | 0 | ��������� | 0 |
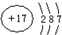 ����1mol X �������ᄃ���к��й��ۼ���ĿΪ4NA��2.408��1024
����1mol X �������ᄃ���к��й��ۼ���ĿΪ4NA��2.408��1024��2��Z���������ɵĻ�����ĵ���ʽΪ
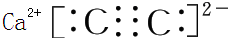
��3��28g Y ��ȫȼ�շų���������283.0kJ��д�� Y ȼ�յ��Ȼ�ѧ����ʽ2CO��g��+O2��g��=2CO2��g������H=-566.0kJ/mol
��4�����W��Ԫ������������Ӧ��ˮ���������ͼ��ʾת����ϵ����Ӧ�����������������ԣ���$\stackrel{��}{��}$��$\stackrel{��}{��}$��
��д�����ڸ�������ˮ��Ӧ�Ļ�ѧ����ʽ3Fe+4H2O$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2��C+H2O��g��$\frac{\underline{\;����\;}}{\;}$CO+H2
�����W��Ԫ�صļ��⻯�K������ˮ����Ҫԭ����NH3��H2O�����γ���������⻯����������Թ���һ��ȼ�ϵ�أ��������Һ��KOH���为���ĵ缫��ӦʽΪ2NH3-6e-+6OH-=N2+6H2O��
 ��ѧ�������-100��ĵ����ºϳ���һ����X��������ͺ˴Ź������������е���ԭ�ӵĻ�ѧ����û�����𣬸��ݷ����������˷��ӵ����ģ�ͣ���ͼ������˵���в���ȷ���ǣ�������
��ѧ�������-100��ĵ����ºϳ���һ����X��������ͺ˴Ź������������е���ԭ�ӵĻ�ѧ����û�����𣬸��ݷ����������˷��ӵ����ģ�ͣ���ͼ������˵���в���ȷ���ǣ�������| A�� | ����X��������ļ�����ȣ�Xȼ��ʱ���ĵ��������� | |
| B�� | ������̼ԭ�ӵĻ�ѧ������2�� | |
| C�� | �����е���ԭ�ӷֲ����������ֱ��ֱ���� | |
| D�� | ��������C-C����Ҳ��C=C |
| A�� | M��������G�� | |
| B�� | N��������G�� | |
| C�� | ��һ��Ӧ����Ҫ����һ���ܷ��� | |
| D�� | ��Ӧ�������֮�ʹ��������������֮�� |
| A�� | CH3COOD��C2H5OD | B�� | CH3COONa��C2H5OD��HOD | ||
| C�� | CH3COONa��C2H5OH��HOD | D�� | CH3COONa��C2H5OD��H2O |
| A�� | 1L 1mol•L-1����������Һ�к��е�Al3+Ϊ2NA | |
| B�� | pH=1��H2SO4��Һ�к���H+����ĿΪ0.2NA | |
| C�� | ������5.6 g Fe��������Ũ���ᷴӦ��ת�Ƶ��ӵ���ĿΪ0.3NA | |
| D�� | 27g���������������NaOH��Һ��Ӧ��ת�Ƶ��ӵ���Ŀ��Ϊ3NA |
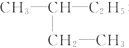 ��
��
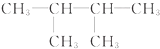 ��
��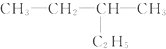
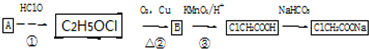
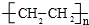 ��
��