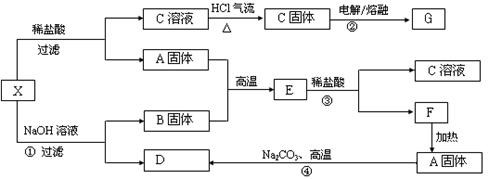
��Ŀ����
�ڸ����£�Fe��ˮ�����ɷ�����Ӧ��ijͬѧ����ͼװ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ����������ɸ�����Fe��ˮ�����ķ�Ӧʵ�飮
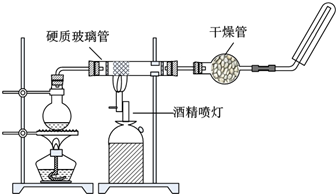
��ش��������⣺
��1��Ӳ�ʲ������з�Ӧ�Ļ�ѧ����ʽΪ______���÷�Ӧ����������______��
��2���Թ����ռ�������______�����Ҫ��A�������ܴ���ȼ���壬�����Ը��������______��ֻ�������д����������̣�����һ������Ŀ����______��

��ش��������⣺
��1��Ӳ�ʲ������з�Ӧ�Ļ�ѧ����ʽΪ______���÷�Ӧ����������______��
��2���Թ����ռ�������______�����Ҫ��A�������ܴ���ȼ���壬�����Ը��������______��ֻ�������д����������̣�����һ������Ŀ����______��
��1������ˮ��������������ԭ��Ӧ��3Fe+4H2O
Fe3O4+4H2�����ݻ��ϼ��������ж��������ͻ�ԭ���������Ļ��ϼ����ߣ�����������ԭ����ˮ����Ļ��ϼ۽��ͣ�ˮ����������
�ʴ�Ϊ��3Fe+4H2O
Fe3O4+4H2��ˮ��
��2�������ı�ը������4.0%��75.6%������˵�������ĺ�����������Χ�ڵĻ��ͻ�����ը����ˣ���ȼ֮ǰҪ���鴿�ȣ���ֹ��ȼʱ������������������ը��
�ʴ�Ϊ���������鴿����ֹ��ȼʱ������������������ը��
| ||
�ʴ�Ϊ��3Fe+4H2O
| ||
��2�������ı�ը������4.0%��75.6%������˵�������ĺ�����������Χ�ڵĻ��ͻ�����ը����ˣ���ȼ֮ǰҪ���鴿�ȣ���ֹ��ȼʱ������������������ը��
�ʴ�Ϊ���������鴿����ֹ��ȼʱ������������������ը��

��ϰ��ϵ�д�
�����Ŀ