��Ŀ����
ij�о�С����CaCl2��H2Ϊԭ�ϣ���ͼ�Ʊ� +1��Ca�Ļ����������ֲ�����ֻ�����ֻ�������ң���Ԫ����ɷ���������������иơ���Ԫ�ص����������ֱ�Ϊ52.36%��46.33%���������ҵ�ˮ��Һ�����ԡ���ش��������⣺
��1�����о�С���Ƿ�ɹ��Ƶ� +1��Ca�Ļ���� ����ǡ������Ļ�ѧʽ�� ��
��2������ˮ��Ӧ�ɵ�H2���仯ѧ����ʽ�� ����Ӧ������Һ���ᾧ�ɵõ�һ�־��壬�仯ѧʽΪCaCl2�� xCa(OH)2�� 12H2O��Ϊȷ��x��ֵ�������ʵ�鷽�� ��
��3���ڼ��������£��ҵ�ˮ��Һ��Ũ����MnO2��Ӧ�����ӷ���ʽ�� ���ҵ�ˮ��Һ��Fe��Ӧ���õ���Һ���ȶ����������Һ�Ĵ�ʩ�� ��
��4����д��һ������Ϊ���ܵõ�CaCl�Ļ�ѧ����ʽ����CaCl2Ϊԭ�ϣ� ��
��1���� ��1�֣�CaHCl ��2�֣�
��2��2CaHCl + 2H2O = CaCl2 + Ca(OH)2 + 2H2����2�֣�
ȡ������ϡHNO3�ܽ��ֳɶ��ȷݣ�����һ�ݼ���Na2CO3��Һ���õ�CaCO3���������غ����n(Ca2+)����һ�ݼ���AgNO3��Һ���õ�AgCl���������غ����n(Cl-)�� ����ʽ�������xֵ�� ��3�֣�����������Ҳ���֣�
��3�֣�����������Ҳ���֣�
��3��2Cl- + MnO2 + 4H+�� Mn2+ + Cl2��+ 2H2O ��2�֣�
����FeCl2��Һ�����ԣ����������۷�ֹ���� ��2�֣�
��4��Ca + CaCl2 = 2CaCl
���������������1����������иơ���Ԫ�ص����������ֱ�Ϊ52.36%��46.33%����������100%С�������������л�������Ԫ�أ�����������Ϊ��100%��52.36%��46.33%��1.31%����ˣ�������ȷ�����������ʵĻ�ѧʽ��
N(Ca)�UN(Cl)�UN(H)��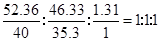
���Եó���ѧʽΪ��CaHCl�����Կ���û�еõ���ν����һ�۵ĸơ�
��2������ˮ��Ӧ�ɵ�H2��˵���Ƿ�������Ԫ�ص�����������ԭ��Ӧ��
2CaHCl + 2H2O = CaCl2 + Ca(OH)2 + 2H2��
ȡ������ϡHNO3�ܽ��ֳɶ��ȷݣ�����һ�ݼ���Na2CO3��Һ���õ�CaCO3���������غ����n(Ca2+)����һ�ݼ���AgNO3��Һ���õ�AgCl���������غ����n(Cl-)�� ����ʽ�������xֵ��
��3���ӵ�һ���п���֪����CaCl2��H2��CaHCl��HCl
2Cl- + MnO2 + 4H+��Mn2+ + Cl2��+ 2H2O
�ҵ�ˮ��Һ��Fe��Ӧ���õ���FeCl2��Һ�����ȶ���һ�����ױ������е��������������Ƕ��������ӻᷢ��ˮ�⣬�������Һ�Ĵ�ʩ�DZ���FeCl2��Һ�����ԣ����������۷�ֹ����
��4��������ǿ�Ļ�ԭ������ԭ����Ca + CaCl2 = 2CaCl
���㣺�������ʵIJⶨ��

CO�dz����Ļ�ѧ���ʣ��й������ʺ�Ӧ�õ��о����¡�
��1����ͬѧ��ΪCO���Ա����Ը��������Һ����ΪCO2���������ʵ����֤��ͬѧ�IJ����Ƿ���ȷ��������������ͼ��������������ҩƷ�����ƻ�ѧʽ���Ӷ����������ƣ��������Բ���Ҳ���Բ�������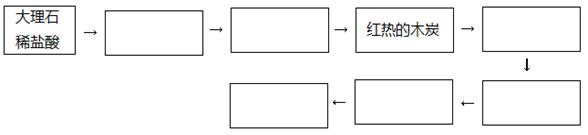
��2��CO��������һ�������¿��Ժϳ����ʻ�����[Fe(CO)5]�������ʿ�������Ǧ���͵ķ���������һ��dz��ɫҺ�壬�۵㡪20.5�棬�е�103�棬�����ڱ����л��ܼ���������ˮ���ܶ�1.46��1.52g/cm3���ж�������ʱ����Fe2(CO)9��60�淢����ȼ�����ʻ��������Ʊ�ԭ�����£�
Fe(s)+5CO(g) Fe(CO)5(g)
Fe(CO)5(g)
������˵����ȷ���� ��
| A������������Ӧԭ�����Ʊ��ߴ��� |
| B���Ʊ�Fe(CO)5Ӧ�ڸ��������������½��� |
C����ӦFe(s)+5CO(g) Fe(CO)5(g)��ƽ�ⳣ������ʽΪ Fe(CO)5(g)��ƽ�ⳣ������ʽΪ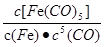 |
| D��Fe(CO)5Ӧ�ܷ⡢�������ܹⲢ����������ˮҺ������ |
�۽�һ������Fe(CO)5�ı���Һ��������������Ƭ�̡�ȡ��������Һ��ȫȼ�գ��õ�30.58gCO2��
5.4gH2O��1.6g����ɫ��ĩ������ɫ��ĩ�Ļ�ѧʽΪ ����������Һ��Fe(CO)5��Fe2 (CO)9�����ʵ���֮��Ϊ .
ij���������ʵ���о�ʱ��������Ũ�Ⱦ�Ϊ1.0mol?L-1 ��Ba(OH)2��H2SO4��Һ��
��.����ͬѧ������Ba(OH)2��Һʱ��ֻ�ҵ��ڿ����б�¶�Ѿõ�Ba(OH)2��8H2O�Լ�����Է���������315����������ȡ�Լ���ˮ�н������ܽ⣬�ձ��д��ڴ���δ���
��1���û�ѧ����ʽ����δ���������ԭ��
��2��ijͬѧ���Ba(OH)2��8H2O��283K��293K��303Kʱ���ܽ�ȣ�g/100g H2O���ֱ�Ϊ2.5��3.9��5.6���ݴ���Ϊ����ʹ�ô�����Ba(OH)2��8H2O����������Ҳ�������1.0 mol?L-1Ba(OH)2��Һ���������ǣ�
��.��18 mol?L-1��Ũ��������450 mL 1.0 mol?L-1ϡ���ᡣ
(3)ʵ��ʱ������ȡŨ��������Ϊ mL�����õ���Ҫ��������Ͳ���ձ����������� ��
(4)������Һʱ�����ݵIJ��������ǣ�
(5)ʵ�������������ʹ������ҺŨ��ƫ�ߵ���
| A��ϴ����ȡŨ�������Ͳ2��3�Σ�����ϴ��Һת������ƿ�� |
| B������ʱ���ӿ̶��� |
| C����ҡ�Ⱥ������¼�ˮ���̶��� |
| D����ˮϴ������ƿδ���� |
��״���£�1���ˮ�����ܽ�500�����HCl���塣����1 Lˮ��ͨ���״���µ�448LHCl���壬����������ȫ�ܽ⡣
��1����������Һ�ܶ�Ϊ1.2 g/cm3������Һ�к�HCl���ʵ���Ũ��Ϊ ��
��2���Ӹ���Һ��ȡ��10mLŨ�����ܽ���ˮ���Ƴ�500mL��Һ�����ƺ��ϡ��Һ�к�HCl���ʵ���Ũ��Ϊ ��
��3������Ũ������������ϡ����ʱ�����������У�ʹ��ǰ�������Ƿ�©Һ�������� �����ƹ����У����Ũ��ƫ�͵IJ���������_______________��ѡ�����в�������ţ���
| A������ƿ����ˮϴ��δ�Ӹ��� |
| B����Ͳ������ˮϴ��δ���� |
| C�����ձ���Ũ������������ƿ��δ��ˮϴ���ձ������������м�ˮ���̶� |
| D���ý�ͷ�ι�������ƿ�м�ˮʱ�����������̶��ߣ������⽺ͷ�ιܴ�ƿ������������Һʹʣ����Һ���ɴ�̶��� |
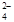 + 2Fe2+ + 4H+
+ 2Fe2+ + 4H+ + 6 Fe2+ + 14 H+ ��2 Cr3+ + 6 Fe3+ + 7 H2O
+ 6 Fe2+ + 14 H+ ��2 Cr3+ + 6 Fe3+ + 7 H2O