��Ŀ����
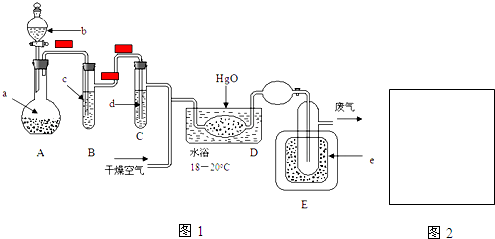
19���������Ʊ���Ϊ��ҵ�Σ���Ư�ס���Ƶȷ���Ӧ�ù㷺����ľ̿��Ũ���ᡢˮ��ͭΪԭ�����ɵ�һ��������������Ʒ�Ӧ�Ʊ��������Ƶ�װ����ͼ��ʾ��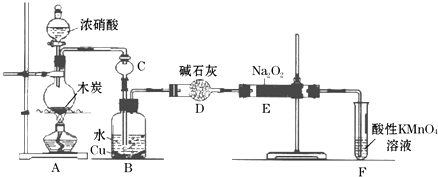
��֪�������£���2NO+Na2O2�T2NaNO2
�����������£�NO��NO2-������MnO4-��Ӧ����NO3-��Mn2+��5NO2-+2MnO4-+6H+�T5NO3-+2Mn2++3H2O
��1��A�й۲쵽���������к���ɫ�������� �� �У����ɫ�������ɣ�
��2��װ��B�У�ʹCu�ܽ�����ӷ���ʽ��3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O��
��3��װ��C�������Ƿ�ֹ������װ��F������������δ��Ӧ��NO��
��4������װ��D����E�в����������������и�����Na2CO3��NaOH�����ѧʽ��
��5����ַ�Ӧ��ijͬѧ���ʵ���E��NaNO2�ĺ������м�⣮��ȡE�й���2g����ȫ�ܽ����Ƴ���Һ100mL��ȡ��25mL��Һ��0.100mol/L����KMnO4��Һ���еζ������ʲ���KMnO4��Ӧ��������KMnO4��Һ20mL������Ʒ���������Ƶ���������Ϊ69.0%��
���� װ��A����Ũ�����̼���ȷ����ķ�Ӧ����Ӧ���ɶ��������Ͷ�����̼��ˮ��װ��B����Aװ�����ɵĶ���������ˮ��Ӧ���������һ�������������ͭ��Ӧ��������ͭ��һ��������ˮ��ͨ��װ��E�еĹ�����������һ��������������̼�����ͨ�����Ը��������Һ��ȥʣ��һ��������ֹ��Ⱦ������
��1��A�����ɶ���������
��2������������ˮ��Ӧ�������ᣬͭ����ϡ���ᷴӦ��
��3�����ƿ�ɷ�ֹ���������Ը��������Һ������δ��Ӧ��NO��
��4��������̼�������Ʒ����ķ�Ӧ����̼���ƺ�������ˮ��������Ʒ�Ӧ�����������ƣ�
��5�����ݷ���ʽ5NO2-+2MnO4-+6H+=5NO3-+2Mn2++3H2O������������Ƶ����ʵ�����Ȼ������Ʒ���������Ƶ�����������
��� �⣺װ��A����Ũ�����̼���ȷ����ķ�Ӧ����Ӧ���ɶ��������Ͷ�����̼��ˮ��װ��B����Aװ�����ɵĶ���������ˮ��Ӧ���������һ�������������ͭ��Ӧ��������ͭ��һ��������ˮ��ͨ��װ��E�еĹ�����������һ��������������̼�����ͨ�����Ը��������Һ��ȥʣ��һ��������ֹ��Ⱦ������
��1������װ��ͼ��֪��A��Ũ������ľ̿����������ԭ��Ӧ����CO2��NO2�Լ�ˮ�����A�й۲쵽�������к���ɫ�������ɣ��ʴ�Ϊ���к���ɫ�������� �� �У����ɫ�������ɣ�
��2��NO2����ˮ����NO�����ᣬ��������������ܰѽ���ͭ��������װ��B�з�Ӧ�����ӷ���ʽ��3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O���ʴ�Ϊ��3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O��
��3��NO2��������ˮ��ˮ��Ӧ������װ��C�������Ƿ�ֹ������NO��������Ʒ�Ӧ����ʣ�࣬�����ŷ��������������Ⱦ����NO�ܱ����Ը��������Һ��������װ��F����������δ��Ӧ��NO���ʴ�Ϊ����ֹ����������δ��Ӧ��NO��
��4�����ɵ�NO�к���CO2��ˮ���������߾�����������Ʒ�Ӧ������ʯ�ҿ�������CO2��ˮ�������������û��Dװ�ã���E�в����������������и�����Na2CO3��NaOH���ʴ�Ϊ��Na2CO3��NaOH��
��5�����ĸ�����ص����ʵ�����0.1mol/L��0.02L=0.002mol������ݷ���ʽ5NO2-+2MnO4-+6H+=5NO3-+2Mn2++3H2O��֪���������Ƶ����ʵ�����0.002mol��$\frac{5}{2}$=0.005mol����ԭ��Ʒ���������Ƶ����ʵ�����0.005mol��$\frac{100mL}{25mL}$=0.02mol��������Ϊ0.02mol��69g/mol=1.38g��������Ʒ���������Ƶ���������$\frac{1.38g}{2g}$��100%=69.0%���ʴ�Ϊ��69.0%��
���� ���⿼����������ʡ�β��������ʵ���������ơ�ʵ�鷽������������Լ����ʺ����ⶨ��ע��ʵ������еķ�Ӧ������������ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | �����ܽ� | B�� | �Ȼ�����Һ | C�� | �Ȼ�����Һ | D�� | ����ͭ��Һ |
| A�� | ����Ӽ䷢������ײ������Ч��ײ | |
| B�� | ����Ӧ��Ũ�Ȼ�ӿ췴Ӧ���ʣ�ԭ���������˻���Ӱٷ������Ӷ�ʹ��Ч��ײ�������� | |
| C�� | ���ȷ�Ӧ�������¶ȣ�v������v����С | |
| D�� | ���������ķ�Ӧ�ﵽƽ�������ѹǿ��ƽ����ܲ��ƶ� |
���������ϡ�
��1�������������ƣ�Na2S2O4����һ�ְ�ɫ��ĩ��������ˮ���������Ҵ���
��2��2Na2S2O4+4HCl=4NaCl+S��+3SO2��+2H2O��Na2S2O3+2HCl=2NaCl+S��+SO2��+H2O��
���Ʊ�������
75��ʱ�������ƺʹ�������Ҵ�ˮ��Һ�У�ͨ��SO2���з�Ӧ������䷴Ӧ����ʽ��
HCOONa+1Na2CO3+4SO2��=2Na2S2O4+3CO2+��
��ȴ��40��50�棬���ˣ����Ҵ�ϴ�ӣ������Ƶ�Na2S2O4��
��Na2S2O4�����ʡ�
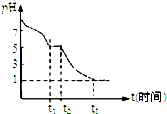
��1��Na2S2O4��Һ�ڿ������ױ�����������С��ⶨ0.050mol/LNa2S2O4��Һ�ڿ�����pH�仯��ͼ��ʾ��0��t1����Ҫ������HSO3-��0��t1�η�����Ӧ�����ӷ�Ӧ����ʽΪ2S2O42-+O2+2H2O=4HSO3-��
t3ʱ��Һ����Ҫ��������SO42-��
��2��������������Na2S2O4������ȫ�ֽ⣬�õ��������Na2SO3��Na2S2O3������ΪSO2���ѧʽ����
�������ʵ����֤������Na2S2O3���ڣ�����±������ݣ�
����ѡ����Լ���ϡ���ᡢϡ���ᡢBaCl2��Һ��KMnO4��Һ��
| ʵ�鲽�裨��Ҫ��д������������̣� | Ԥ�ڵ�ʵ������ͽ��� |
| ȡ������ȫ�ֽ�Ĺ���������Թ��У�����ϡ���� | ���е���ɫ����������Na2S2O3���� |
| A�� | ��������������ı䷴Ӧ;�������ͷ�Ӧ����Ҫ�Ļ�ܡ���������������Ч��ײ�����������ѧ��Ӧ�������� | |
| B�� | ��������Ӧ��Ũ�ȣ���λ�����������������λ�����Ч��ײ�����������ѧ��Ӧ�������� | |
| C�� | ���������¶ȣ�����Ӱٷ��������ҷ��Ӽ����ײƵ����ߡ���Ч��ײ�����������ѧ��Ӧ�������� | |
| D�� | �����Ӵ���ϵѹǿ�������С������Ӱٷ������ӡ���λ����Ļ������Ŀ���ӡ���Ч��ײ�����������ѧ��Ӧ�������� |
H2��g��+Cl2��g���T2HCl��g����H=-Q1kJ/mol��
H2��g��+Br2��g���T2HBr��g����H=-Q2kJ/mol��
�й�������Ӧ��������ȷ���ǣ�������
| A�� | Q1��Q2 | |
| B�� | �����������������ڷ�Ӧ�������� | |
| C�� | ����1molHCl����ʱ�ų�Q1���� | |
| D�� | ���Q1��Q2���Ͽ�Cl-Cl����Ҫ�������ȶϿ�Br-Br����Ҫ�������� |
| A�� | ����������������10 mL | B�� | c��NH4+��=c��Cl-�� | ||
| C�� | ����������������10 mL | D�� | c��NH4+����c��Cl-�� |
| A�� | ����ֻ�ı䷴Ӧ������Ӧ���� | |
| B�� | ����ͨ�����߷�Ӧ�Ļ�����ӿ췴Ӧ���� | |
| C�� | �����ܹ��ı䷴Ӧ�ķ�Ӧ�� | |
| D�� | �������ܸı䷴Ӧ���ת���� |