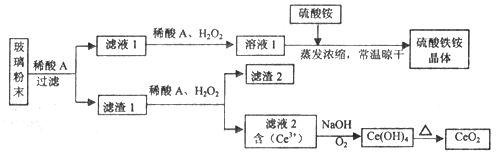
��Ŀ����
����Ŀ��һ���¶��£���ͬ������ĸ����������зֱ���������ĸ�ƽ�⣺
��N2(g)+3H2(g)![]() 2NH3(g) K1
2NH3(g) K1
��H2(g)+I2(g)![]() 2HI(g) K2
2HI(g) K2
��2NO2(g)![]() N2O4(g) K3
N2O4(g) K3
��C(s)+H2O(g)![]() CO(g)+H2(g) K4
CO(g)+H2(g) K4
��������и��⣺
��1��д����Ӧ����ƽ�ⳣ���ı���ʽK4=____________��
��2��������ͬ�¶��µ�����ƽ�⣺
�� 2N2(g)+6H2(g)![]() 4NH3(g) K5
4NH3(g) K5
��2HI(g)![]() H2(g)+I2(g) K6
H2(g)+I2(g) K6
��K5=__ ��K6=_ ������K1��K2��K3��K4��ʾ��
��3����ƽ������NO2���������Ϊa��ijʱ���ټ���һ������N2O4����ʱ�ԣ����� �ԣ��棩���ٴδﵽƽ���NO2��������� a�����������������������
��������1��K4=![]() ��
��
��2��K5=K12��K6=1/K2��
��3���ԣ��������ԣ��棩������
��������
�����������1����ѧƽ�ⳣ������������ƽ��Ũ��ϵ�����ݵĻ��뷴Ӧ��ƽ��Ũ��ϵ�����ݵĻ��ı�ֵ������Ӧ����ƽ�ⳣ���ı���ʽK4=![]() ��
��
��2����֪��N2(g)+3H2(g)![]() 2NH3(g) ������5��2N2(g)+6H2(g)
2NH3(g) ������5��2N2(g)+6H2(g)![]() 4NH3(g) ��K5=K12�����������̻��棬ƽ�ⳣ����Ϊ����������2HI(g)
4NH3(g) ��K5=K12�����������̻��棬ƽ�ⳣ����Ϊ����������2HI(g) ![]() H2(g)+I2(g)��ƽ�ⳣ��K6=1/K2��
H2(g)+I2(g)��ƽ�ⳣ��K6=1/K2��
��3����ƽ������NO2���������Ϊa��ijʱ���ټ���һ������N2O4��ƽ�������ƶ�����ʱ�ԣ�����<�ԣ��棩���ټ���һ������N2O4���൱������ѹǿ��ƽ�������ƶ����ٴδﵽƽ���NO2���������<a��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����ѧ�����������Ź㷺��Ӧ�ã����ж�����ϵ��ȷ����
ѡ�� | ��ѧ���� | ʵ��Ӧ�� |
A | SO2���л�ԭ�� | Ư��ֽ�� |
B | HF���������� | �ڲ����Ͽ��֣� |
C | ���Ľ������ǿ���� | ��������������Ũ���� |
D | FeCl3��Һ����Cu��Ӧ | ʴ��ͭ�������·�� |
A. A B. B C. C D. D
����Ŀ����һ�ܱ�������ͨ��A��B��C�������壬����һ���¶ȣ���t1��t4 sʱ��ø����ʵ�Ũ��������������ж���ȷ����( )
�ⶨʱ��/s Ũ�� | t1 | t2 | t3 | t4 |
c(A)/(mol��L��1) | 6 | 3 | 2 | 2 |
c(B)/(mol��L��1) | 5 | 3.5 | 3 | 3 |
c(C)/(mol��L��1) | 1 | 2.5 | 3 | 3 |
A. ��t3 sʱ��Ӧ�Ѿ�ֹͣ
B. t3��t4 s�������淴Ӧ���ʲ����
C. �������з����ķ�ӦΪA(g)��B(g) C(g)
D. ��t2��t3 s��A��ƽ����Ӧ����Ϊ![]() mol/(L��s)
mol/(L��s)