جâؤ؟ؤعبف
،¾جâؤ؟،؟SnCl4تاز»ضض¼«ز×ث®½âµؤ»¯؛دخثüµؤضئ±¸ذèزھشعخقث®µؤجُ¼دآ£¬أـ±صµؤ×°ضأضذ½ّذذ،£بôسأ³£¹و(دµح³µؤجه»½د´َ)·½·¨½ّذذ¶àتµرéب±µم±ب½د¶à£¬دآح¼²ةسأخ¢ذحتµرé½ّذذSnCl4 µؤضئ±¸£¬½â¾ِءث³£¹و·½·¨µؤ±×¶ث(¼؛ضھ£؛SnCl4µؤبغµمخھ£33،و£¬·ذµمخھ114.1،و)،£
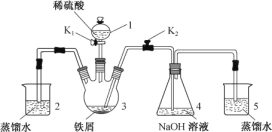
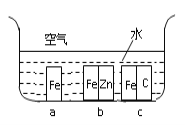
(1)½«زر¸ةشïµؤ¸÷²؟·ضزائ÷°´ح¼ء¬½س؛أ؛َ£¬ذèزھ½ّذذµؤ²ظ×÷خھ________________________،£
(2)Vذخ¹ـµؤ×÷سأتا________________________________________________________،£
(3)دآءذثµ·¨ضذصب·µؤتا_____
A.زائ÷Aµؤأû³ئخھصôءَةصئ؟
B.خھءث³ن·ض¸ةشïآبئّ£¬إ¨ءٍثلµؤجه»س¦´َسعاٍإفµؤجه»
C.²ظ×÷ت±س¦دبµخ¼سإ¨رخثل£¬ت¹صûج××°ضأؤع³نآْ»ئآجة«ئّجه£¬شظسأأ؛ئّµئ¼سبب
D.ةْ³ةµؤSnCl4صôئّ¾ہنب´¾غ¼¯شعؤ¥؟ع¾كض§¹ـضذ
E.خ¢ذح¸ةشï¹ـضذµؤتش¼ء؟ةزشتا¼îت¯»ز،¢خهرُ»¯¶ء×»ٍخقث®آب»¯¸ئµب
(4)تµرéضذ0.59خء£حêب«·´س¦ضئµأ1.03g SnCl4£¬شٍ¸أتµرéµؤ²ْآتخھ_____________(¼ئثم½ل¹û±£ءôز»خ»ذ،ت)،£
(5)SnCl4سِ°±¼°ث®صôئّµؤ·´س¦تاضئ×÷رجؤ»µ¯µؤشہي£¬·´س¦µؤ»¯ر§·½³جت½خھ_______________،£
(6)¸أخ¢ذحتµرéµؤسإµمتا________________________________________________(بخذ´ء½جُ)،£
،¾´ً°¸،؟¼ى²é×°ضأئّأـذش سأ×÷»؛³هئ؟»ٍ°²ب«ئ؟ CDE 93.9% SnCl4+4NH3+4H2O=Sn(OH)4+4NH4Cl ²ْآت¸ك£¬¶ش»·¾³خغب¾ذ،£¬تµرéز©ئ·سأء؟½دةظ£¨بخذ´ء½µم£¬؛دہي¼´؟ة£©
،¾½âخِ،؟
¸كأجثل¼ط؛حإ¨رخثل·´س¦ةْ³ةآبئّ£¨؛¬سذآب»¯ا⣩£¬¾¹إ¨ءٍثل¸ةششع¾كض§تش¹ـؤع¸كخآ¼سببجُ¼دآآبئّسëخء£·´س¦ةْ³ةSnCl4µؤصôئû£¬¾¹ہنؤ¹ـہنؤ£¬شع¾كض§تش¹ـؤعتص¼¯SnCl4£¬خ´·´س¦µؤآبئّ¾µ¼¹ـسأتµرé×°ضأخ²²؟µؤ½تھاâرُ»¯ؤئبـز؛µؤأقاٍ½ّذذخüتص،£
(1)سةسعSnCl4تاز»ضض¼«ز×ث®½âµؤ»¯؛دخثüµؤضئ±¸ذèزھشعخقث®µؤجُ¼دآ£¬ءيحâضئب،µؤآبئّسذ¶¾£¬¸كخآدآسëخء£·´س¦ةْ³ةSnCl4µؤصôئû£¬زٍ´ثشع½ّذذضئ±¸تµرéا°£¬زھ¼ى²é×°ضأµؤئّأـذش£¬·ہض¹؟صئّضذث®صôئّ½ّبë¼°·ہض¹آبئّ½ّبë´َئّ£»
(2)¸ù¾ف·ضخِ£¬شع¾كض§تش¹ـؤع¸كخآ¼سببجُ¼دآآبئّسëخء£·´س¦ةْ³ةSnCl4µؤصôئû£¬µ¼ضآتµرé×°ضأؤعر¹ا؟شِ´َ£¬زٍ´ثVذخ¹ـئًµ½°²ب«ئ؟»ٍئً»؛³ه×÷سأ£»
(3) A.¸ù¾فح¼ت¾زائ÷Aµؤ¹¹شى£¬²»تاصôءَةصئ؟£»
B. سةسعSnCl4تاز»ضض¼«ز×ث®½âµؤ»¯؛دخخھءث³ن·ض¸ةشïآبئّ£¬إ¨ءٍثلµؤجه»س¦±£ض¤خقآبئّؤـض±½سح¨¹اٍإف£¬²»ذèزھجه»´َسعاٍإف£»
C. SnCl4تاز»ضض¼«ز×ث®½âµؤ»¯؛دخ²ظ×÷ت±س¦دبµخ¼سإ¨رخثل£¬ت¹صûج××°ضأؤع³نآْ»ئآجة«ئّجه£¬½«تµرé×°ضأؤعµؤ؟صئّب«²؟¸د³ِ£¬±£ض¤¸ةشïخقث®µؤ»·¾³£¬شظسأأ؛ئّµئ¼سبب£»
D. ¸ù¾ف·ضخِ£¬شع¾كض§تش¹ـؤع¸كخآ¼سببجُ¼دآآبئّسëخء£·´س¦ةْ³ةSnCl4µؤصôئû£¬¾¹ہنؤ¹ـہنؤ£¬شع¾كض§تش¹ـؤعتص¼¯SnCl4£»
E. خ¢ذح¸ةشï¹ـضذµؤض÷زھ×÷سأتا·ہض¹ث®صôئّ½ّبëؤ¥؟ع¾كض§تش¹ـ£¬·ہض¹SnCl4ث®½â£¬ءيح⻹؟ةزشسأسعخüتصخ´·´س¦µؤسذ¶¾ئّجهآبئّ£¬زٍ´ثتش¼ء؟ةزشتا¼îت¯»ز،¢خهرُ»¯¶ء×»ٍخقث®آب»¯¸ئµبؤـ¹»خüث®؛حخüتصآبئّµؤ¸ةشï¼ء£»
(4)¸ù¾ف·´س¦Sn+Cl2![]() SnCl4£¬¼ئثم³ِةْ³ةSnCl4µؤہيآغضµ£¬شظ¸ù¾ف²ْآت=
SnCl4£¬¼ئثم³ِةْ³ةSnCl4µؤہيآغضµ£¬شظ¸ù¾ف²ْآت=![]() ½ّذذ¼ئثم£»
½ّذذ¼ئثم£»
(5)SnCl4سِ°±¼°ث®صôئّµؤ·´س¦تاضئ×÷رجؤ»µ¯µؤشہيتا£؛SnCl4+4NH3+4H2O=Sn(OH)4+4NH4Cl£»
(6)¸أخ¢ذحتµرéµؤسإµمتا²ْآت¸ك£¬¶ش»·¾³خغب¾ذ،£¬تµرéز©ئ·سأء؟½دةظ£»
(1)سةسعSnCl4تاز»ضض¼«ز×ث®½âµؤ»¯؛دخثüµؤضئ±¸ذèزھشعخقث®µؤجُ¼دآ£¬ءيحâضئب،µؤآبئّسذ¶¾£¬¸كخآدآسëخء£·´س¦ةْ³ةSnCl4µؤصôئû£¬زٍ´ثشع½ّذذضئ±¸تµرéا°£¬زھ¼ى²é×°ضأµؤئّأـذش£¬·ہض¹؟صئّضذث®صôئّ½ّبë¼°·ہض¹آبئّ½ّبë´َئّ£»
¹ت´ً°¸خھ£؛¼ى²é×°ضأئّأـذش£»
(2)¸ù¾ف·ضخِ£¬شع¾كض§تش¹ـؤع¸كخآ¼سببجُ¼دآآبئّسëخء£·´س¦ةْ³ةSnCl4µؤصôئû£¬µ¼ضآتµرé×°ضأؤعر¹ا؟شِ´َ£¬زٍ´ثVذخ¹ـئًµ½°²ب«ئ؟»ٍئً»؛³ه×÷سأ£»
¹ت´ً°¸خھ£؛سأ×÷»؛³هئ؟»ٍ°²ب«ئ؟£»
(3) A. ¸ù¾فح¼ت¾زائ÷Aµؤ¹¹شى£¬²»تاصôءَةصئ؟£¬¹تA´يخَ£»
B. سةسعSnCl4تاز»ضض¼«ز×ث®½âµؤ»¯؛دخخھءث³ن·ض¸ةشïآبئّ£¬إ¨ءٍثلµؤجه»س¦±£ض¤خقآبئّؤـض±½سح¨¹اٍإف£¬²»ذèزھجه»´َسعاٍإف£¬¹تB´يخَ£»
C. SnCl4تاز»ضض¼«ز×ث®½âµؤ»¯؛دخ²ظ×÷ت±س¦دبµخ¼سإ¨رخثل£¬ت¹صûج××°ضأؤع³نآْ»ئآجة«ئّجه£¬½«تµرé×°ضأؤعµؤ؟صئّب«²؟¸د³ِ£¬±£ض¤¸ةشïخقث®µؤ»·¾³£¬شظسأأ؛ئّµئ¼سبب£¬¹تCصب·£»
D. ¸ù¾ف·ضخِ£¬شع¾كض§تش¹ـؤع¸كخآ¼سببجُ¼دآآبئّسëخء£·´س¦ةْ³ةSnCl4µؤصôئû£¬¾¹ہنؤ¹ـہنؤ£¬شع¾كض§تش¹ـؤعتص¼¯SnCl4£¬¹تDصب·£»
E. خ¢ذح¸ةشï¹ـضذµؤض÷زھ×÷سأتا·ہض¹ث®صôئّ½ّبëؤ¥؟ع¾كض§تش¹ـ£¬·ہض¹SnCl4ث®½â£¬ءيح⻹؟ةزشسأسعخüتصخ´·´س¦µؤسذ¶¾ئّجهآبئّ£¬زٍ´ثتش¼ء؟ةزشتا¼îت¯»ز،¢خهرُ»¯¶ء×»ٍخقث®آب»¯¸ئµبؤـ¹»خüث®؛حخüتصآبئّµؤ¸ةشï¼ء£¬¹تEصب·£»
¹ت´ً°¸خھCDE£»
(4)ةْ³ةSnCl4µؤ·´س¦خھ£؛Sn+2Cl2![]() SnCl4£¬0.59خµؤخïضتµؤء؟=
SnCl4£¬0.59خµؤخïضتµؤء؟=![]() =0.004mol£¬شٍ¸ù¾ف·´س¦£¬ةْ³ةSnCl4µؤخïضتµؤء؟=0.004mol£¬¼´SnCl4µؤضتء؟=0.004mol،ء261g/mol=1.097g£¬
=0.004mol£¬شٍ¸ù¾ف·´س¦£¬ةْ³ةSnCl4µؤخïضتµؤء؟=0.004mol£¬¼´SnCl4µؤضتء؟=0.004mol،ء261g/mol=1.097g£¬
شٍSnCl4²ْآت=![]() =
=![]() =93.9%£¬
=93.9%£¬
¹ت´ً°¸خھ£؛93.9%£»
(5)SnCl4سِ°±¼°ث®صôئّµؤ·´س¦تاضئ×÷رجؤ»µ¯µؤشہيتا£؛SnCl4+4NH3+4H2O=Sn(OH)4+4NH4Cl£¬
¹ت´ً°¸خھ£؛SnCl4+4NH3+4H2O=Sn(OH)4+4NH4Cl£»
(6)¸أخ¢ذحتµرéµؤسإµمتا²ْآت¸ك£¬¶ش»·¾³خغب¾ذ،£¬تµرéز©ئ·سأء؟½دةظ£¬
¹ت´ً°¸خھ£؛²ْآت¸ك£¬¶ش»·¾³خغب¾ذ،£¬تµرéز©ئ·سأء؟½دةظ£¨بخذ´ء½µم£¬؛دہي¼´؟ة£©£»

 ²¹³ند°جâ½ثصدµءذ´ً°¸
²¹³ند°جâ½ثصدµءذ´ً°¸ ر§ء·؟ى³µµہ؟عثمذؤثمثظثمجىجىء·دµءذ´ً°¸
ر§ء·؟ى³µµہ؟عثمذؤثمثظثمجىجىء·دµءذ´ً°¸،¾جâؤ؟،؟رادُُ£آب![]() £¬بغµم£؛
£¬بغµم£؛![]() £¬·ذµم£؛
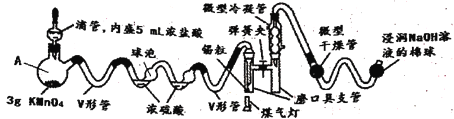
£¬·ذµم£؛![]() خھ؛ى؛ضة«ز؛جه»ٍ»ئة«ئّجه£¬¾كسذ´ج±ا¶ٌ³ôخ¶£¬سِث®¾çءزث®½âةْ³ةµھµؤء½ضضرُ»¯خïسëآب»¯ا⣬ز×بـسعإ¨ءٍثل،£تاسذ»ْخï؛د³ةضذµؤضطزھتش¼ء£¬³£؟ةسأسع؛د³ةاه½à¼ء،¢´¥أ½¼ء¼°ضذ¼نجهµب،£تµرéتز؟ةسةآبئّسëز»رُ»¯µھشع³£خآ³£ر¹دآ؛د³ة،£ئنضئ±¸×°ضأبçح¼ثùت¾
خھ؛ى؛ضة«ز؛جه»ٍ»ئة«ئّجه£¬¾كسذ´ج±ا¶ٌ³ôخ¶£¬سِث®¾çءزث®½âةْ³ةµھµؤء½ضضرُ»¯خïسëآب»¯ا⣬ز×بـسعإ¨ءٍثل،£تاسذ»ْخï؛د³ةضذµؤضطزھتش¼ء£¬³£؟ةسأسع؛د³ةاه½à¼ء،¢´¥أ½¼ء¼°ضذ¼نجهµب،£تµرéتز؟ةسةآبئّسëز»رُ»¯µھشع³£خآ³£ر¹دآ؛د³ة،£ئنضئ±¸×°ضأبçح¼ثùت¾![]() ئنضذIII،¢IVضذ¾ùخھإ¨ءٍثل
ئنضذIII،¢IVضذ¾ùخھإ¨ءٍثل![]() £؛
£؛
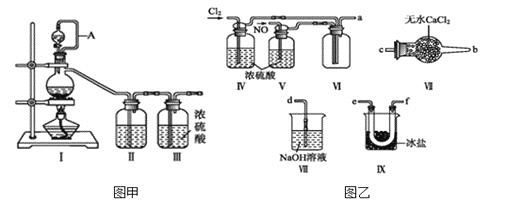
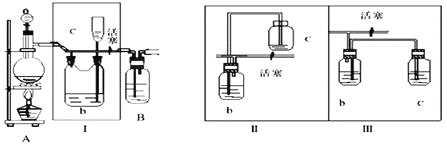
(1)سأح¼¼×ضذ×°ضأضئ±¸´؟¾»¸ةشïµؤشءدئّ£¬²¹³ندآ±يضذثùب±ةظµؤز©ئ·،£
| ×°ضأ¢ٌ | ×°ضأ¢ٍ | |
| ةصئ؟ضذ | ·ضز؛آ©¶·ضذ | |
ضئ±¸´؟¾» |
| إ¨رخثل |
|
ضئ±¸´؟¾»NO | Cu | د،دُثل |
|
(2)½«ضئµأµؤNO؛ح![]() ح¨بëح¼زز¶شس¦×°ضأضئ±¸NOCl،£
ح¨بëح¼زز¶شس¦×°ضأضئ±¸NOCl،£
![]() ×°ضأء¬½سث³ذٍخھ
×°ضأء¬½سث³ذٍخھ![]() ________
________![]() °´ئّء÷×ش×َدٍسز·½دٍ£¬سأذ،ذ´×ضؤ¸±يت¾
°´ئّء÷×ش×َدٍسز·½دٍ£¬سأذ،ذ´×ضؤ¸±يت¾![]() ،£
،£
![]() ×°ضأ¢ô،¢¢ُ³؟ة½ّز»²½¸ةشïNO،¢
×°ضأ¢ô،¢¢ُ³؟ة½ّز»²½¸ةشïNO،¢![]() ح⣬»¹؟ةزشح¨¹¹غ²ىئّإفµؤ¶àةظµ÷½عء½ضضئّجهµؤء÷ثظ،£
ح⣬»¹؟ةزشح¨¹¹غ²ىئّإفµؤ¶àةظµ÷½عء½ضضئّجهµؤء÷ثظ،£
![]() سذبثبدخھ؟ةزش½«×°ضأ¢ôضذإ¨ءٍثل؛د²¢µ½×°ضأ¢ُضذ£¬³·³×°ضأ¢ô£¬ض±½س½«NO،¢
سذبثبدخھ؟ةزش½«×°ضأ¢ôضذإ¨ءٍثل؛د²¢µ½×°ضأ¢ُضذ£¬³·³×°ضأ¢ô£¬ض±½س½«NO،¢![]() ح¨بë×°ضأ¢ُضذ£¬ؤمح¬زâ´ث¹غµمآً£؟_______
ح¨بë×°ضأ¢ُضذ£¬ؤمح¬زâ´ث¹غµمآً£؟_______![]() جî،°ح¬زâ،±»ٍ،°²»ح¬زâ،±
جî،°ح¬زâ،±»ٍ،°²»ح¬زâ،±![]() £¬شزٍتا___________،£
£¬شزٍتا___________،£
![]() تµرé؟ھت¼µؤت±؛ٍ£¬دبح¨بëآبئّ£¬شظح¨بëNO£¬شزٍخھ________________________،£
تµرé؟ھت¼µؤت±؛ٍ£¬دبح¨بëآبئّ£¬شظح¨بëNO£¬شزٍخھ________________________،£
(3)سذبثبدخھ¶àسàµؤآبئّ؟ةزشح¨¹دآءذ×°ضأشفت±´¢´و؛َشظہûسأ£¬اëر،شٌ؟ةزشسأ×÷آبئّµؤ´¢ئّµؤ×°ضأ ______£»
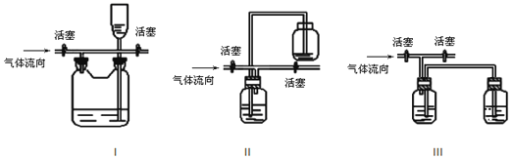
(4)×°ضأ¢÷خüتصخ²ئّت±£¬NOCl·¢ةْ·´س¦µؤ»¯ر§·½³جت½خھ_______________________،£
(5)سذبثبدخھ×°ضأ¢÷ضذاâرُ»¯ؤئبـز؛ض»ؤـخüتصآبئّ؛حNOCl£¬²»ؤـخüتصNO£¬¾¹²éشؤ×تءد·¢دضسأ¸كأجثل¼طبـز؛؟ةزشخüتصNOئّجه£¬زٍ´ثشع×°ضأ¢÷اâرُ»¯ؤئبـز؛ضذ¼سبë¸كأجثل¼ط£¬·´س¦²ْةْ؛عة«³ءµي£¬ذ´³ِ¸أ·´س¦µؤہë×س·½³جت½£؛__________________________________،£
(6)ضئµأµؤNOClضذ؟ةؤـ؛¬سذةظء؟![]() شسضت£¬خھ²â¶¨²ْئ·´؟¶ب½ّذذبçدآتµرé£؛³ئب،
شسضت£¬خھ²â¶¨²ْئ·´؟¶ب½ّذذبçدآتµرé£؛³ئب،![]() رùئ·بـسع
رùئ·بـسع![]() بـز؛ضذ£¬¼سب뼸µخ
بـز؛ضذ£¬¼سب뼸µخ![]() بـز؛×÷ض¸ت¾¼ء£¬سأ×مء؟دُثلثل»¯µؤ
بـز؛×÷ض¸ت¾¼ء£¬سأ×مء؟دُثلثل»¯µؤ![]() بـز؛µخ¶¨ضء²ْةْש؛ىة«³ءµي£¬دû؛ؤ
بـز؛µخ¶¨ضء²ْةْש؛ىة«³ءµي£¬دû؛ؤ![]() بـز؛
بـز؛![]() ،£شٍ¸أرùئ·µؤ´؟¶بخھ__________
،£شٍ¸أرùئ·µؤ´؟¶بخھ__________![]() ±£ءô1خ»ذ،ت
±£ءô1خ»ذ،ت![]()
،¾جâؤ؟،؟ؤ³ح¬ر§¶شCl2سëKIبـز؛µؤ·´س¦½ّذذءثتµرéج½¾؟،£·´س¦×°ضأبçدآ£؛
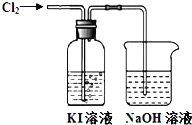
ح¨بëآبئّز»¶خت±¼ن£¬KIبـز؛±نخھ»ئة«،£¼جذّح¨بëآبئّز»¶خت±¼ن؛َ£¬بـز؛»ئة«حتب¥£¬±نخھخقة«،£¼جذّح¨بëآبئّ£¬×î؛َبـز؛±نخھا³»ئآجة«،£
£¨1£©Cl2سëNaOHبـز؛·´س¦µؤ»¯ر§·½³جت½تا___،£
£¨2£©KIبـز؛±نخھ»ئة«ثµأ÷آبئّ¾كسذµؤ___ذش£¬¸أ·´س¦µؤہë×س·½³جت½تا___،£
£¨3£©زرضھI2+I-![]() I3-£¬I2،¢I3-شعث®ضذ¾ù³ت»ئة«،£خھب·¶¨»ئة«بـز؛µؤ³ة·ض£¬½ّذذءثزشدآتµرé،£
I3-£¬I2،¢I3-شعث®ضذ¾ù³ت»ئة«،£خھب·¶¨»ئة«بـز؛µؤ³ة·ض£¬½ّذذءثزشدآتµرé،£
¢ظتµرébµؤؤ؟µؤتا___،£
¢ع¸ù¾فتµرéaضذ£¬ث®²مضذ؛¬سذµؤء£×سسذ___،£
²ظ×÷ | تµرéدضدَ | |
a | ب،2،«3mL»ئة«بـز؛£¬¼سبë×مء؟CCl4£¬صٌµ´¾²ضأ | CCl4²م³ت×د؛ىة«£¬ث®²مدشا³»ئة« |
b | ب،2،«3mL±¥؛حµâث®£¬¼سبë×مء؟CCl4£¬صٌµ´¾²ضأ | CCl4²م³ت×د؛ىة«£¬ث®²م¼¸½üخقة« |
¢غتµرéaضذث®بـز؛رصة«±نا³µؤشزٍتا___،£
¢ـخھ±£ض¤تµرéµؤرد½÷ذش£¬شعتµرéa،¢bµؤ»ù´،ةد£¬ذè²¹³نز»¸ِتµر飬¸أتµرéخھ___،£
£¨4£©ح¨بëآبئّ£¬بـز؛سة»ئة«±نخھخقة«£¬تازٍخھآبئّ½«I2رُ»¯£¬زرضھ1molCl2؟ةرُ»¯0.2molI2£¬¸أ·´س¦µؤ»¯ر§·½³جت½تا___،£
£¨5£©¸ù¾فةدتِتµر飬اëش¤²âدٍµي·غ-KIبـز؛ضذ³ضذّح¨بëآبئّ£¬؟ةؤـ¹غ²ىµ½µؤدضدَخھ___،£
£¨6£©بـز؛×îضص±نخھا³آجة«µؤشزٍتا___،£