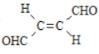��Ŀ����
����Ŀ�������仯������;�dz��㷺��
��1����֪������ԭұ��ʱ�����еģ���֪��
(a) 3Fe2O3(s)��CO(g) ![]() 2Fe3O4(s)��CO2(g)
2Fe3O4(s)��CO2(g) ![]() H = a kJ��mol��1
H = a kJ��mol��1
(b) Fe2O3(s)��3CO(g) ![]() 2Fe(s)��3CO2(g)
2Fe(s)��3CO2(g) ![]() H =b kJ��mol��1
H =b kJ��mol��1
(c) Fe3O4(s)��CO(g) ![]() 3FeO(s)��CO2 (g)
3FeO(s)��CO2 (g) ![]() H = c kJ��mol��1
H = c kJ��mol��1
��������Ӧ(a)ƽ�ⳣ������ʽΪK=___________________��
�ڷ�ӦFeO(s)��CO(g) ![]() Fe(s)��CO2(g)
Fe(s)��CO2(g) ![]() H =__________kJ��mol��1���ú�a��b��c�Ĵ���ʽ��ʾ����
H =__________kJ��mol��1���ú�a��b��c�Ĵ���ʽ��ʾ����
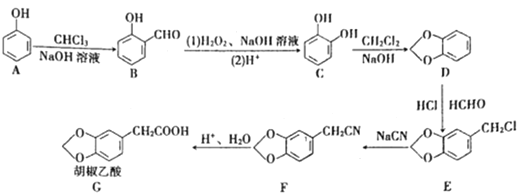
��2��������ұ�����ķ����� ��600��1000��ʱ������ڵ�Fe2O3ұ������װ��ʾ��ͼ��ͼ�����÷����������������⣬��һ��ͻ�����ŵ���__________________________________�����ʱ���������ĵ缫��ӦΪ____________��

��3����������Ȼ���пɷ������ⸯʴ��������ʴ�����ⸯʴ�ĸ����ĵ缫��ӦʽΪ_________����ͼ��ʾ����������a��b��c���ֲ�ͬ�Ļ����У�������ʴ�������ɴ�С��˳���ǣ�����ĸ��_____________��

���𰸡�![]() (3b-a-2c)/6 ������CO2 2O2--4e-=O2�� Fe-2e-=Fe2+ c>a>b
(3b-a-2c)/6 ������CO2 2O2--4e-=O2�� Fe-2e-=Fe2+ c>a>b
��������
(1)�ٹ������ʲ�д��ƽ�ⳣ������ʽ�У�
�����ݸ�˹���ɣ�[3(b)-(a)2(c)]/6����FeO(s)+CO(g)![]() Fe(s)+CO 2(g)��
Fe(s)+CO 2(g)��
(2)��ԭ��ұ���������л����CO2���壬�Ի������ƻ�����ͼ�п���������ڵ�Fe2O3ұ����ʱ��O2-������ʧȥ��������O2��
(3) ���ⸯʴ�����ڸ���ʧ���������������ӣ�����̼����ԭ��أ��ӿ������ĸ�ʴ������п����ԭ��أ�Zn��������������Fe��������Fe�ĸ�ʴ��
(1)�ٷ�Ӧ(a)Ϊ3Fe2O3(s)��CO(g)![]() 2Fe3O4(s)��CO2(g)��ƽ�ⳣ������ʽΪ��
2Fe3O4(s)��CO2(g)��ƽ�ⳣ������ʽΪ��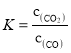 ���ʴ�Ϊ��
���ʴ�Ϊ��![]()
�ڸ��ݸ�˹���ɣ�[3(b)-(a)-2(c)]/6�ɵã�FeO(s)��CO(g)![]() Fe(s)��CO2(g)����
Fe(s)��CO2(g)����![]() H =(3b-a-2c)/6kJ��mol��1���ʴ�Ϊ��(3b-a-2c)/6��
H =(3b-a-2c)/6kJ��mol��1���ʴ�Ϊ��(3b-a-2c)/6��
(2) ��ԭ��ұ���������л����CO2���壬�Ի������ƻ���������ڵ�Fe2O3ұ����ʱ��������CO2��O2-������ʧȥ��������O2�������������ĵ缫��ӦΪ��2O2--4e-=O2�����ʴ�Ϊ��������CO2��2O2--4e-=O2����
(3)���ⸯʴ�����ڸ���ʧ���������������ӣ��为���ĵ缫��ӦʽΪ��Fe-2e-=Fe2+������̼����ԭ��أ��ӿ������ĸ�ʴ������п����ԭ��أ�Zn������Fe��������Fe�ĸ�ʴ������������ʴ�������ɴ�С��˳���ǣ�c>a>b���ʴ�Ϊ��Fe-2e-=Fe2+��c>a>b��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�������£��úͦ�4��������ϩ��(����ͼ)�ǹ���ά����E����Ҫ���ʣ����������в���ȷ����( )
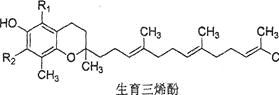
�� �� �� �� | |
R1 | CH3 CH3 H H |
R2 | CH3 H CH3 H |
A.���ͦ�����������ϩ�ӻ�Ϊͬϵ��ºͦ�����������ϩ�ӻ�Ϊͬ���칹��
B.4��������ϩ�Ӿ���ʹ���Ը��������Һ��ɫ
C.4��������ϩ�Ӷ�������ˮ
D.1mol��������ϩ������ˮ������Ӧ����������������3molBr2
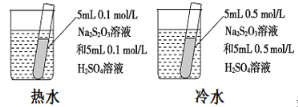
����Ŀ����������̼���������������ڲ�ͬ��ʵ�������½��з�Ӧ������ڲ�ͬʱ��(t)�ڲ����������(V)����������ͼ��ʾ������ͼʾ����ʵ������������˵����һ������ȷ������ ��
��� | ��Ӧ���� | c(HCl) / mol��L-1 | ��Ӧ�¶� / �� | ����״̬ | |
1 | a | 30 | ��ĩ״ | ||
2 | b | 30 | ��ĩ״ | ||
3 | c | 2.5 | ��״ | ||
4 | d | 2.5 | 30 | ��״ |
A. ��4��ʵ��ķ�Ӧ��������
B. ��1��ʵ���������Ũ�ȿ������
C. ��2��ʵ��������Ũ�ȿ��ܵ���2.5mol/L
D. ��3��ʵ��ķ�Ӧ�¶ȵ���30 ��