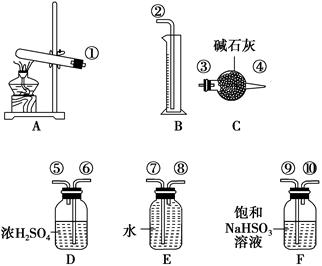
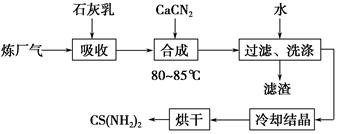
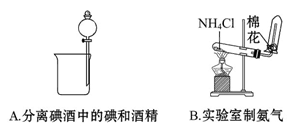
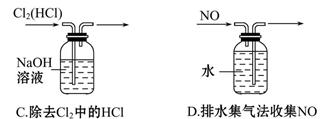
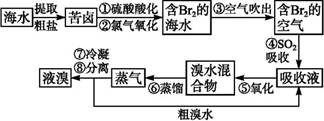
��Ŀ����
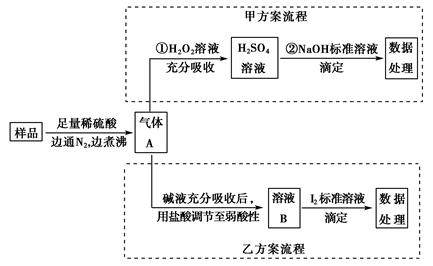
̼���ơ���������Ӻ���(aNa2CO3��bH2O2)����Ư�ס�ɱ�����á�ʵ�����á����������Ʊ������ʵ�ʵ�鲽�����£�
��1����ȡ����̼�����ܽ���һ����ˮ�������ƿ�У��ټ��������ȶ���(MgCl2��Na2SiO3)��������ȡ�
��2����������30%��H2O2��Һ�ڽ���״̬�µ�����ƿ�У���15 �����ҷ�Ӧ1 h��
��3������Ӧ��Ϻ��ټ���������ˮ�Ҵ������á��ᾧ�����ˡ�����ò�Ʒ��
(1)��1���У��ȶ�����ˮ��Ӧ�������ֳ�����������仯ѧ����ʽΪ___________________________________________________________��
(2)��2���У���Ӧ����Ϊ15 �����ҿɲ�ȡ�Ĵ�ʩ��_____________________
___________________________________________________��
(3)��3���У���ˮ�Ҵ���������____________________________________��
(4)H2O2�ĺ����ɺ�����Ʒ�����ӡ��ֳ�ȡm g(Լ0.5 g)��Ʒ��������й�������ˮ���Ƴ�250 mL��Һ��ȡ25.0 mL����ƿ�У�����ϡ�����ữ������c mol��L��1 KMnO4��Һ�ζ����յ㡣
������250 mL��Һ����IJ����������ձ�������������Ͳ________��________��
�ڵζ��յ�۲쵽��������______________________________________��

(5)��ģ�������ⶨ��Ʒ��̼���Ƶĺ�����װ������ͼ��ʾ(���Ⱥ̶�װ������ȥ)��ʵ�鲽�����£�
����1������ͼ��ʾ��װ���������װ�������ԡ�
����2��ȷ��ȡ(4)��������Һ50 mL����ƿ�С�
����3��ȷ��ȡ40.00 mLԼ0.2 mol��L��1 NaOH��Һ���ݣ��ֱ�ע���ձ�����ƿ�С�
����4������K1��K2���رջ���K3����ͨ�뵪��һ��ʱ��ر�K1��K2����K3������Һ©������ƿ�м���10 mL 3 mol��L��1������Һ��
����5����������ƿ�е�Һ����ڣ���������һ��ʱ�䡣
����6����K1�ٻ���ͨ�뵪��һ��ʱ�䡣
����7������ƿ�м������ָʾ������c1 mol��L��1 H2SO4����Һ�ζ����յ㣬����H2SO4����ҺV1 mL��
����8����ʵ�鲽��1��7�ظ����Ρ�
�ٲ���3�У�ȷ��ȡ40.00 mL NaOH��Һ����Ҫʹ�õ�������________��
�ڲ���1��7�У�ȷ�����ɵĶ�����̼������������Һ��ȫ���յ�ʵ�鲽����________(�����)��
��Ϊ�����Ʒ��̼���Ƶĺ��������貹���ʵ����______________________��
��1����ȡ����̼�����ܽ���һ����ˮ�������ƿ�У��ټ��������ȶ���(MgCl2��Na2SiO3)��������ȡ�
��2����������30%��H2O2��Һ�ڽ���״̬�µ�����ƿ�У���15 �����ҷ�Ӧ1 h��
��3������Ӧ��Ϻ��ټ���������ˮ�Ҵ������á��ᾧ�����ˡ�����ò�Ʒ��
(1)��1���У��ȶ�����ˮ��Ӧ�������ֳ�����������仯ѧ����ʽΪ___________________________________________________________��
(2)��2���У���Ӧ����Ϊ15 �����ҿɲ�ȡ�Ĵ�ʩ��_____________________
___________________________________________________��
(3)��3���У���ˮ�Ҵ���������____________________________________��
(4)H2O2�ĺ����ɺ�����Ʒ�����ӡ��ֳ�ȡm g(Լ0.5 g)��Ʒ��������й�������ˮ���Ƴ�250 mL��Һ��ȡ25.0 mL����ƿ�У�����ϡ�����ữ������c mol��L��1 KMnO4��Һ�ζ����յ㡣
������250 mL��Һ����IJ����������ձ�������������Ͳ________��________��
�ڵζ��յ�۲쵽��������______________________________________��

(5)��ģ�������ⶨ��Ʒ��̼���Ƶĺ�����װ������ͼ��ʾ(���Ⱥ̶�װ������ȥ)��ʵ�鲽�����£�
����1������ͼ��ʾ��װ���������װ�������ԡ�
����2��ȷ��ȡ(4)��������Һ50 mL����ƿ�С�
����3��ȷ��ȡ40.00 mLԼ0.2 mol��L��1 NaOH��Һ���ݣ��ֱ�ע���ձ�����ƿ�С�
����4������K1��K2���رջ���K3����ͨ�뵪��һ��ʱ��ر�K1��K2����K3������Һ©������ƿ�м���10 mL 3 mol��L��1������Һ��
����5����������ƿ�е�Һ����ڣ���������һ��ʱ�䡣
����6����K1�ٻ���ͨ�뵪��һ��ʱ�䡣
����7������ƿ�м������ָʾ������c1 mol��L��1 H2SO4����Һ�ζ����յ㣬����H2SO4����ҺV1 mL��
����8����ʵ�鲽��1��7�ظ����Ρ�
�ٲ���3�У�ȷ��ȡ40.00 mL NaOH��Һ����Ҫʹ�õ�������________��
�ڲ���1��7�У�ȷ�����ɵĶ�����̼������������Һ��ȫ���յ�ʵ�鲽����________(�����)��
��Ϊ�����Ʒ��̼���Ƶĺ��������貹���ʵ����______________________��
��(1)MgCl2��Na2SiO3��2H2O===2NaCl��Mg(OH)2����H2SiO3����(2)15 ��ˮԡ����ˮԡ��(3)����̼���Ƶ��ܽ��(�����ھ�������)
(4)��250 mL����ƿ����ͷ�ιܡ�����Һ�ʷۺ�ɫ��30 s����ɫ
(5)�ټ�ʽ�ζ��ܡ���1,5,6������H2SO4����Һ�ζ�NaOH��Һ��Ũ��
(4)��250 mL����ƿ����ͷ�ιܡ�����Һ�ʷۺ�ɫ��30 s����ɫ
(5)�ټ�ʽ�ζ��ܡ���1,5,6������H2SO4����Һ�ζ�NaOH��Һ��Ũ��
��(1)�����ȶ������Ȼ�þ�����ƣ��������Ϻ������������ܻ������˸÷�Ӧ�Ļ�ѧ����ʽΪMgCl2��Na2SiO3��2H2O===Mg(OH)2����H2SiO3����2NaCl��(2)Ҫ����ȶ��ı�����Һ���¶�Ϊ15 �棬�Ϻõķ�������15 ���ˮԡ��(3)��Ӧ��Ϻ�����Һ�м�����ˮ�Ҵ�����Ҫ�����ǽ���̼���Ƶ��ܽ�ȣ���������ᾧ������(4)����250 mL��Һ��Ҫ�Ĺؼ�������250 mL������ƿ��Ϊ������ȷȷ����Һ�����������Ҫ�ձ�����Ͳ������������ͷ�ιܵȣ����������Ը��������Һ�ζ�˫��ˮ����˴ﵽ�յ�ʱ�������ĸ�����ؿ�����ָʾ�����յ�ʱ������Ϊ�������һ�����Ը��������Һ��ɫ�ʷۺ�ɫ���Ұ���Ӳ���ɫ��(5)Ҫȷ��ȡ40.00 mL NaOH��Һ����Ҫ���ȸߵ���������������Ͳ��Ӧ���ü�ʽ�ζ��ܣ�ʵ��ʱ��ͨ�뵪����Ϊ�˷�ֹϵͳ�п�����CO2Ӱ��ʵ���������ͨ������Ϊ�˰����ɵ�CO2ȫ�����ռ����գ����װ����������Ϊ�˷�ֹ���ɵ�CO2�ݳ������������ƿ�е���Һ�ǰ����ɲ��ܽ�����Һ�е�CO2�ϳ�Ȼ���ռ����գ����ʵ�鲽��1��5��6��Ϊ�˱�֤���ɵ�CO2��ȫ���ռ����գ�����ʵ�����ռ���Һ��Ũ���Ǵ�ԼΪ0.2 mol��L��1�����Ҫ��ȷ����ʵ����������Ҫ֪���ռ���Һ��ȷŨ�ȣ������Ҫ�ñ�������Һ�ζ��ռ���Һ��Ũ�ȡ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ