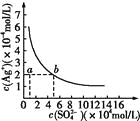
��Ŀ����
����Ŀ����ͼ��ʾΪ��������У�����ͭ��������ᣨHN3�����ɵ�ԭ��أ��ܷ�Ӧ����ʽΪ�� 2Cu+2Cl- +HN3+3H+��2CuCl(s)+N2��+NH4+�����������������
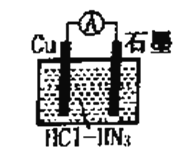
A. ���ӵ�����ΪCu��![]() ��ʯī
��ʯī
B. �����ĵ缫��ӦʽΪCu+Cl�D�De����CuCl(s)
C. ��Ӧһ��ʱ�����������Һ��pH��С
D. ��״���£�����224mL N2ʱ��ͭת�Ƶĵ�����Ϊ0.02NA
���𰸡�C
��������A. ���ܷ�Ӧ����ʽ��֪��Cuʧ��������CuCl������CuΪ������ʯīΪ���������ӵ�����ΪCu��![]() ��ʯī����A��ȷ��B. Cuʧ��������CuCl���缫��ӦʽΪCu+Cl�D�De����CuCl(s)����B��ȷ��C. �����ĵ缫��ӦʽΪHN3��2e����3H��=N2����NH4�����ɵ缫��Ӧʽ��֪����Ӧһ��ʱ�����������Һ��pH����C����D. ���ܷ�Ӧ����ʽ��֪��Cu�Ļ��ϼ۴�0�����ߵ�+1��������1mol����ʱ������2molCu��ת��2mol���ӣ��ڱ�״���£�224mL N2�����ʵ���Ϊ0.01mol��������0.02molCu��Cuת�Ƶĵ�����Ϊ0.02NA����D��ȷ����ѡC��
��ʯī����A��ȷ��B. Cuʧ��������CuCl���缫��ӦʽΪCu+Cl�D�De����CuCl(s)����B��ȷ��C. �����ĵ缫��ӦʽΪHN3��2e����3H��=N2����NH4�����ɵ缫��Ӧʽ��֪����Ӧһ��ʱ�����������Һ��pH����C����D. ���ܷ�Ӧ����ʽ��֪��Cu�Ļ��ϼ۴�0�����ߵ�+1��������1mol����ʱ������2molCu��ת��2mol���ӣ��ڱ�״���£�224mL N2�����ʵ���Ϊ0.01mol��������0.02molCu��Cuת�Ƶĵ�����Ϊ0.02NA����D��ȷ����ѡC��

��ϰ��ϵ�д�
 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
�����Ŀ