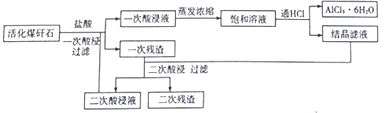
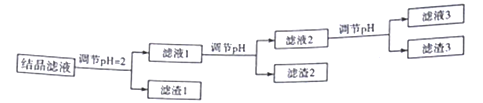
��Ŀ����
����Ŀ��A��B��C��D��E��λ�ڶ����ڵ�����Ԫ�ء���֪�������ȶ��ԣ�HmD��HmC����Cm-��E(m-1)-������ͬ�ĵ��Ӳ�ṹ����A��B��ͬһ���ڣ��ڸ�������������Ԫ���У�A��ԭ�Ӱ뾶���B�����Ӱ뾶��С����A��B������֮����D��������3��������������Ϣ����Ӧ����ѧ�����ش��������⣺
��1��HmDm�ĵ���ʽ_________��
��2��Cm-��E(m-1)-�Ļ�ԭ��ǿ��˳��Ϊ��___���������ӷ��ű�ʾ����֤���仹ԭ��ǿ�������ӷ���ʽΪ______��
��3����E�ĵ���ͨ��A��D�γɵĻ������ˮ��Һ�У��ڳ����·�Ӧ�����ӷ���ʽΪ��_______��
��4�������£��������ʵ���Ũ�ȵ�HmC��Һ��A������������Ӧ��ˮ������Һ�������1:2��ϣ�д���÷�Ӧ�����ӷ���ʽ_____������Һ�����ʺ��еĻ�ѧ��������_____
���𰸡�![]() S2����Cl�� Cl2+S2��=2Cl��+S�� Cl2+2OH��=Cl��+ClO��+H2O H2S+2OH��=S2��+H2O ���Ӽ�
S2����Cl�� Cl2+S2��=2Cl��+S�� Cl2+2OH��=Cl��+ClO��+H2O H2S+2OH��=S2��+H2O ���Ӽ�
��������
�����⣬A��B��ͬһ���ڣ��ڸ�������������Ԫ���У�A��ԭ�Ӱ뾶���B�����Ӱ뾶��С����A��BΪ������A��B������֮����D��������3������A��B��������֮��Ϊ3�ı�������A��B���ڵ������ڣ���AΪNaԪ�ء�BΪAlԪ�أ�D��������Ϊ(11+13)/3=8����DΪ��Ԫ�أ����ȶ��ԣ�HmD>HmC����C��D����ͬһ���壬��D�ķǽ����Ը�ǿ����CΪSԪ�أ�m=2�����ݣ�(2) Cm-��E(m-1)-������ͬ�ĵ��Ӳ�ṹ����EΪClԪ�ء�
(1)m=2��DΪOԪ�أ���HmDmΪH2O2������ʽΪ![]() ��
��
(2)ͬ����Ԫ�ش����ң��ǽ�������ǿ���ǽ�����Cl>S�����ʵ�������Cl2>S�����ʵ�������Խǿ����Ӧ�����ӵĻ�ԭ��Խ�������л�ԭ�ԣ�S2����Cl������ͨ�����ʼ���û���Ӧ��֤����Cl2+S2��=2Cl��+S����
(3)E�ĵ���Ϊ������A��D�γɵĻ������ˮ��ҺΪNaOH��Һ���ڳ������������������Ʒ�Ӧ�����Ȼ��ơ�����������ˮ����Ӧ�����ӷ���ʽΪ��Cl2+2OH��=Cl��+ClO��+H2O ��
(4)�����£��������ʵ���Ũ�ȵ�H2S��Һ��NaOH��Һ�������1��2��ϣ���Ӧ����������ˮ���÷�Ӧ�����ӷ���ʽΪH2S+2OH��=S2��+H2O����Һ���������ƣ��������Ӽ���