��Ŀ����
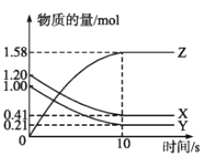
����Ŀ��һ���¶��£���2L���ܱ������У�X��Y��Z������������ʵ�����ʱ��仯��������ͼ��ʾ��
(1)д���÷�Ӧ�Ļ�ѧ����ʽ_________________________��
(2)���㷴Ӧ��ʼ��10s����X��ʾ�ķ�Ӧ������___________��
(3)������������˵��������Ӧ�ﵽƽ��״̬����_________��
a����X��Y�ķ�Ӧ����֮��Ϊ1��1
b�����������X��Ũ�ȱ��ֲ���
c��X��Y��Z��Ũ��֮��Ϊ1��1��2
(4)Ϊʹ�÷�Ӧ�ķ�Ӧ�������ɲ�ȡ�Ĵ�ʩ��_______��
a���ʵ������¶� b��������������� c������һ����Z
���𰸡�X+Y![]() 2Z 0.0395 mol��L-1��s-1 b c
2Z 0.0395 mol��L-1��s-1 b c
��������
��ͼ����֪���淴Ӧ����X��Y�����ʵ�����С��Z�����ʵ�����������X��Y�Ƿ�Ӧ�Z�������l0s��X��Y��Z�����ʵ���Ϊ��ֵ����Ϊ0����10s�ﵽƽ��״̬����Ӧ�ǿ��淴Ӧ���ҡ�n(X)����n(Y)����n(Z)=(1.20-0.41)mol��(1.00-0.21)mol��1.58mol=1��1��2���μӷ�Ӧ�����ʵ����ʵ���֮�ȵ��ڻ�ѧ������֮�ȣ��ʷ�Ӧ��ѧ����ʽΪX(g)+Y(g)2Z(g)��Ȼ����v=![]() ��ƽ����������ȡ�����������֮�ȵ��ڻ�ѧ������֮�������
��ƽ����������ȡ�����������֮�ȵ��ڻ�ѧ������֮�������
(1)������������֪���÷�Ӧ�Ļ�ѧ����ʽΪX(g)+Y(g)2Z(g)��
(2)��Ӧ��ʼ��10s����X��ʾ�ķ�Ӧ������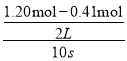 =0.0395mol(Ls)-1��
=0.0395mol(Ls)-1��
(3)a�����ŷ�Ӧ�Ľ��У�X��Y�ķ�Ӧ����֮��ʼ��Ϊ1:1�������ж���ƽ��״̬����a����
b�����������X��Ũ�ȱ��ֲ��䣬����ƽ��������������Ϊƽ��״̬����b��ȷ��
c��X��Y��Z��Ũ��֮��Ϊ1:1:2������ʼ����ת�����йأ������ж���ƽ��״̬����c����
�ʴ�Ϊb��
(4)a���ʵ������¶ȣ���Ӧ���ʼ�С����a����
b�����������������Ũ�ȼ�С����Ӧ���ʼ�С����b����
c������һ����Z��Ũ������Ӧ���ʼӿ죬��cѡ��
�ʴ�Ϊc��

 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�����Ŀ���밴Ҫ��ش��������⣺
(1)�����������Ǵ�����Ⱦ��֮һ���û���̿��һ����̼��ԭ��������ɷ�ֹ������Ⱦ����֪��2C(s)+O2(g)=2CO(g)��H=��22lkJ/mol,
C(s)+O2(g)=CO2(g)��H=��393.5 kJ/mol,
N2(g)+O2(g)=2NO(g)��H=+181 kJ/mol,
��2CO(g)+2NO(g)![]() N2(g)+2CO2(g)��H=__kJ/mol��
N2(g)+2CO2(g)��H=__kJ/mol��
���д�ʩ�ܹ�����˷�Ӧ��NO��ת���ʵ���___(����ĸ���)
a.������������� b.�����¶� c.����CO��Ũ�� d.����NO��Ũ��
(2)���ݻ�Ϊ2L���ܱ������м������̿(����)��NO��������ӦC(s)+2NO(g)![]() N2(g)+CO2(g)��H=��574.5kJ/mol��NO��N2�����ʵ����仯���±���ʾ��
N2(g)+CO2(g)��H=��574.5kJ/mol��NO��N2�����ʵ����仯���±���ʾ��
���ʵ���/mol | T1/�� | T2/�� | |||||
0 | 5 min | 10 min | 15 min | 20 min | 25 min | 30 min | |
NO | 2.0 | 1.20 | 0.70 | 0.70 | 0.50 | 0.40 | 0.40 |
N2 | 0 | 0.40 | 0.65 | 0.65 | 0.75 | 0.80 | 0.80 |
��0~5min�ڣ���NO��ʾ�ĸ÷�Ӧ���ʦ�(NO)=__________���������µ�ƽ�ⳣ��K=___________(����2λС��)��
�ڵ�15min���¶ȵ�����T2�����ݱ仯���ϱ���ʾ����T1___________T2(�>������<����=��)��
(3)�ڻ�ѧ�����в���K2CrO4Ϊָʾ������AgNO3����Һ�ζ���Һ��Cl��������Ag+��CrO42������ש��ɫ������ָʾ����ζ��յ㡣����Һ��Cl��ǡ�ó�����ȫ(Ũ�ȵ���1.0��10��6mol��L��1)ʱ����Һ��c(Ag+)Ϊ__mol��L��1����ʱ��Һ��c(CrO42��)����____mol��L��1��(��֪Ksp(Ag2CrO4)=2.0��10��12��Ksp(AgCl)=2.0��10��10)��