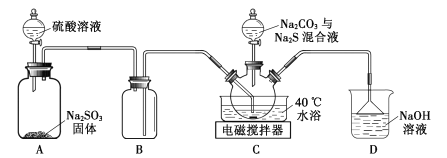
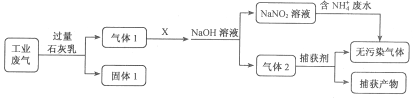
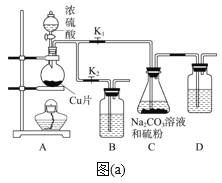
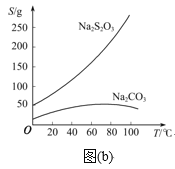
ЬтФПФкШн
ЁОЬтФПЁПаТжЦТШЫЎКЌгаCl2ЁЂH2OЁЂHClOЁЂH+ЁЂClЃЕШСЃзгЃЌЮЊМьбщЦфГЩЗжЃЌФГбаОПадбЇЯАаЁзщзіСЫШчЯТЪЕбщЃЌЧыИљОнЫљзіЪЕбщЃЌАДвЊЧѓЬюПеЁЃ
ЃЈ1ЃЉШЁЩйСПаТжЦТШЫЎгкЪдЙмжаЃЌМгШыЬМЫсИЦЗлФЉЃЌЗЂЯжгаЦјХнВњЩњЃЌдђЫЕУїЦ№зїгУ
ЕФГЩЗжЪЧHClЃЌHClБэЯжГі адЁЃ
ЃЈ2ЃЉШЁЩйСПаТжЦТШЫЎгкЪдЙмжаЃЌМгШыAgNO3ШмвКЃЌЗЂЯжгаАзЩЋГСЕэВњЩњЃЌдђЦ№зїгУЕФЪЧ ЁЃ
ЃЈ3ЃЉШЁЩйСПаТжЦТШЫЎгкЪдЙмжаЃЌМгШывЛПщКьжНЃЌЗЂЯжКмПьЭЪЩЋЃЌдђЦ№зїгУЕФЪЧ ЁЃ
ЃЈ4ЃЉШЁЩйСПаТжЦТШЫЎгкЪдЙмжаЃЌМгШыFeCl2ШмвКЃЌЗЂЯжКмПьБфЛЦЃЌЦ№зїгУЕФГЩЗжЪЧCl2ЃЌЫЕУїТШЦјОпга адЁЃ
ЁОД№АИЁПЃЈ1ЃЉЫсад ЃЈ2ЃЉCl- (3) HClO ЃЈ4ЃЉЧПбѕЛЏ
ЁОНтЮіЁП
ЃЈ1ЃЉбЮЫсгыЬМЫсИЦЗДгІЩњГЩТШЛЏИЦКЭЖўбѕЛЏЬМЦјЬхЃЌБэЯжбЮЫсЕФЫсадЃЛ
ЃЈ2ЃЉбЮЫсгыAgNO3ШмвКЗДгІЩњГЩТШЛЏвјГСЕэЃЌ![]() ЃЛ
ЃЛ
ЃЈ3ЃЉДЮТШЫсОпгаЦЏАзадЃЛЃЈ4ЃЉFeCl2ШмвКгыТШЦјЗДгІЩњГЩТШЛЏЬњЃЌТШЦјЪЧбѕЛЏМСЃЛ
ЃЈ1ЃЉбЮЫсгыЬМЫсИЦЗДгІЩњГЩТШЛЏИЦКЭЖўбѕЛЏЬМЦјЬхЃЌHClБэЯжГіЫсадЃЛ
ЃЈ2ЃЉбЮЫсгыAgNO3ШмвКЗДгІЩњГЩТШЛЏвјГСЕэЃЌ![]() ЃЌаТжЦТШЫЎгкЪдЙмжаЃЌМгШыAgNO3ШмвКЃЌЗЂЯжгаАзЩЋГСЕэВњЩњЃЌдђЦ№зїгУЕФЪЧCl-ЃЛ
ЃЌаТжЦТШЫЎгкЪдЙмжаЃЌМгШыAgNO3ШмвКЃЌЗЂЯжгаАзЩЋГСЕэВњЩњЃЌдђЦ№зїгУЕФЪЧCl-ЃЛ
ЃЈ3ЃЉДЮТШЫсОпгаЦЏАзадЃЌаТжЦТШЫЎгкЪдЙмжаЃЌМгШыКьжНЃЌЗЂЯжКмПьЭЪЩЋЃЌдђЦ№зїгУЕФЪЧHClOЃЛ
ЃЈ4ЃЉFeCl2ШмвКгыТШЦјЗДгІЩњГЩТШЛЏЬњЃЌТШЦјЪЧбѕЛЏМСЃЌЫЕУїТШЦјОпгаЧПбѕЛЏадЁЃ