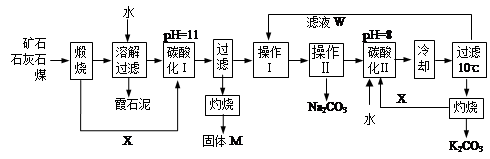
��Ŀ����
�������ȼҵ�еķ�������Ҫ����þ�����������ƵȵĹ����κ�̼���Ρ�ʵ����������Ϊԭ����ȡMgSO4��7H2O���������£�
��֪��(��) Ksp[Mg(OH)2]��6.0��
(��) Fe2+��Fe3+��Al3+��ʼ��������ȫ������pH��Χ����Ϊ��7.1~9.6��2.0~3.7��3.1~4.7
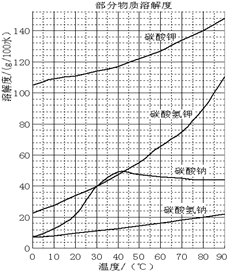
(��) ���ֻ�������ܽ�ȣ�S�����¶ȱ仯������ͼ��
�ش��������⣺
��1���������м�H2SO4��Һ����pHΪ1��2�Լ���һ����е�Ŀ���ǣ�������
��2������Һ��Mg2+��Ũ��Ϊ6 mol/L����ҺpH�������ſ��ܲ���Mg(OH)2������
��3���ڶ��ι�����Ҫ���Ƚ��У���Ҫԭ����������������������Ҫ�ɷ����� ��
��4������Һ���л��MgSO4��7H2O�����ʵ���������Ϊ��������Һ���м����������ڹ��ˣ��ó�������������������Ũ�������½ᾧ���ݹ��ˡ�ϴ�ӵò�Ʒ��
��5������õ�MgSO4��7H2O����Ϊ24.6 g����������к�þ[��Mg(OH)2��]�İٷֺ���Լ ��MgSO4��7H2Oʽ��Ϊ246��
��15�֣�
��1�����Mg2+�Ľ�ȡ�ʣ�2�֣�
��2��8��2�֣�
��3���¶Ƚϸ�ʱ������þ�η�������ף��������CaSO4��2H2O�ܽ��С����2�֣�
Al(OH)3��Fe(OH)3��CaSO4��2H2O ��2�֣�
��4��NaOH��Һ��2�֣� ������м�����ϡ���ᣨ2�֣�
��5��20.0%��3�֣�
�������������������ͼ���٢ڢ�������ܽ⣬�ܷ�����Һ�������ʣ���Ҫ��H2SiO3�Ͳ��ܽ���������ݽ�Fe2�� ������Fe3�� �Ա�ֲ���ȥ������Fe (OH)3��Al (OH)3��MgSO4��NaCl��Һ������Һ��õ���Ʒ����ͨ�������߷�Ӧ���ʣ����Mg2�� �Ľ����ʣ��𰸣����Mg2+�Ľ�ȡ�ʣ���Ksp[Mg(OH)2]��6.0�� =c(Mg2��)c2(OH�D )�ã�c2(OH�D )= 6.0��
=c(Mg2��)c2(OH�D )�ã�c2(OH�D )= 6.0�� /6=1.0��10-12��c(OH�D )=1.0��10�D6mol/L,PH
/6=1.0��10-12��c(OH�D )=1.0��10�D6mol/L,PH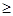 8���𰸣�8���Ǵ�ͼ�ж���������CaSO4��2H2O�ܽ��С������Һ��������ף��𰸣��¶Ƚϸ�ʱ������þ�η�������ף��������CaSO4��2H2O�ܽ��С���� Al(OH)3��Fe(OH)3��CaSO4��2H2O������Һ���к���MgSO4��NaCl��Ҫ��NaCl�����ȥ��Ҫ�Ƚ�Mg2�� �γ�Mg(OH)2���������˺�NaCl��ȥ��Ȼ���H2SO4��������MgSO4��Ȼ������Ũ�������½ᾧ�����ˡ�ϴ�ӡ�������þ��塣�𰸣�NaOH��Һ��������м�����ϡ�����MgSO4��7H2O����Ϊ24.6 g����0.1mol,��0.1molMg (OH)2Ϊ5.8 g��5.8g/29g��100%=20.0%���𰸣�20.0%��
8���𰸣�8���Ǵ�ͼ�ж���������CaSO4��2H2O�ܽ��С������Һ��������ף��𰸣��¶Ƚϸ�ʱ������þ�η�������ף��������CaSO4��2H2O�ܽ��С���� Al(OH)3��Fe(OH)3��CaSO4��2H2O������Һ���к���MgSO4��NaCl��Ҫ��NaCl�����ȥ��Ҫ�Ƚ�Mg2�� �γ�Mg(OH)2���������˺�NaCl��ȥ��Ȼ���H2SO4��������MgSO4��Ȼ������Ũ�������½ᾧ�����ˡ�ϴ�ӡ�������þ��塣�𰸣�NaOH��Һ��������м�����ϡ�����MgSO4��7H2O����Ϊ24.6 g����0.1mol,��0.1molMg (OH)2Ϊ5.8 g��5.8g/29g��100%=20.0%���𰸣�20.0%��
���㣺�ۺ�ʵ���⣬�漰Ԫ�ػ�����֪ʶ��������ԭ�ζ���ָʾ��ѡ����������ʵ�鲽�衢pH���ڵȶ�����ݣ�����ѧ�����ۺ�ʵ�鴦��������

���������ؾ��ں��зḻ��������ʳ����Դ��ʳ�����ճ������еı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
��1�����ⶨ���������´����к�������K+��Ca2+��Mg2+��Fe3+���������ӣ�ij�о���ѧϰС����ʵ�����ᴿNaCl���������£�
���ṩ���Լ�������Na2CO3��Һ������K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba(NO3)2��Һ��75%�Ҵ���Һ��CCl4����������Ʒ��ѡ��
������ȥ��Һ�е�Ca2+��Mg2+��Fe3+��SO42-��ѡ��a���������������Լ������μ�˳������Ϊ
��ֻ�ѧʽ����b�������������� ��
��ϴ�ӳ�ȥNaCl������渽��������KCl��Ӧѡ���Լ��� ����PH��ֽ�ⶨ��Һ��PHֵ�ķ����� ��
��2�����ᴿ��NaCl����500mL��2.5mol��L-1��NaCl��Һ�������������ձ���������ƽ����������ӣ���ҩ�ף��������⣬����Ҫ �����������ƣ���Ӧ��ȡNaCl g
(3)���в����ᵼ������NaCl��ҺŨ��ƫ�ߵ���
| A��������Ϻ�����ҡ�ȣ��ٽ�����ƿ����ʵ��̨�ϣ�����Һ����ڿ̶��ߣ�����������ˮ���̶��ߡ� | |
| B��δ��ϴ���ձ��ڱڵ���Һת������ƿ�� | C������ʱ�����ӿ̶��ߡ� |
| D��ת����Һ֮ǰ������ƿ������������ˮ�� E������ʱ����ƽָ��ָ�����̡� |
����������FeC2O4?2H2O�����������Լ�����Ӱ�������͵�ز�����������﮵���������֪��CO�����Ȼ��٣�PdCl2����Һ��Ӧ���ɺ�ɫ���ٷۡ��ش��������⣺
I����ȤС��Բ��������ķֽ�������ʵ���̽����
��1���������������ͨ��A������ʯ��ˮ��B���Ȼ��٣��۲쵽A�г���ʯ��ˮ������ǣ�B�г��ֺ�ɫ�������ɣ�����������˵������������� ��
��2��̽���ֽ�õ��Ĺ����������Ԫ�صĴ�����ʽ��
���������
����1��________�� ����2��FeO�� ����3��FeO��Fe�����
�����ʵ�鷽��֤������3��
��ѡ�Լ��� 1.0 mol?L��1���ᡢ3% H2O2��0.1 mol?L��1CuSO4��20% KSCN������ˮ��
| ʵ�鲽�� | ��������� |
| ����1 �����Թ��м��������������ټ�������_________________������� | ����Һ��ɫ���Ըı䣬����_______���ɣ���֤���������ʴ��� |
| ����2�� ������1�еõ�����Һ���ˣ���������ˮϴ����ϴ��Һ��ɫ | |
| ����3��ȡ����2�õ��������������Թ��У� �μ�___________________________________ _______________________________________ | __________________________________ ___________________________________ |
II��ij����������Ʒ�к����������ᣨΪ�����ڼ��㣬���������в�����Ͳ�����Ӿ���C2O42�����棩�����õζ����ⶨ����Ʒ��FeC2O4�ĺ������ζ���Ӧ�ֱ��ǣ�5Fe2++MnO4��+8H+=5Fe3+ +Mn2++4H2O��5C2O42��+2MnO4��+16H+=10CO2��+2Mn2++8H2O��
��3��ʵ�鷽�����Ϊ��
�ٽ�ȷ������0.20g����������Ʒ����250 mL��ƿ�ڣ���������2 mol/L��H2SO4��Һ��ʹ��Ʒ�ܽ⣬������70�����ң�������Ũ��Ϊ0.02000 mol/L�ĸ�����ر���Һ�ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ������V1 mL��
���������ζ����Һ�м���������Zn�ۺ�����2 mol/L��H2SO4��Һ�����5��8min����KSCN��Һ�ڵ�ΰ��ϼ�����Һ��ֱ����Һ�����̱�졣����Һ��������һ����ƿ�У�������0.02000 mol/L�ĸ�����ر���Һ�ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ������V2 mL��
����ijС���һ�βⶨ���ݼ�¼���£� V1= 18.90mL��V2=6.20mL���������ݼ���0.20g��Ʒ�У�n��Fe2+��= �� n��C2O42����= ��FeC2O4����������Ϊ ����ȷ��0.01%��FeC2O4��ʽ��Ϊ144��
ij�������д�����CuS���������������P��������������������ʡ�ij��ѧ����С������������̣�ȡ�ÿ���Ϊԭ������CuC12��2H2O���塣
��֪�������£��������ӿ�ʼ�����ͳ�����ȫʱ��pH���±���
| �������� | �������↑ʼ������pH | �������������ȫ��pH |
| Fe2+ | 7.0 | 9.0 |
| Fe3+ | 1.9 | 3.2 |
| Cu2+ | 4.7 | 6.7 |
�ش��������⣺
��1������ٵı��չ�������Ҫͨ����������ʵ�����У���ʹ�ó�����ѧ����������ͼ��ʾװ����ȡ��������д����Ϥ�ķ�������������������ѧ����ʽ��______��______

��2�����ղ�����β���к��е�һ���������γ��������Ⱦ�����β��ͨ�백ˮ�У��ܷ��������Ӧ��д�����п��ܷ���������������ԭ��Ӧ�Ļ�ѧ����ʽ��______��______
��3���������������У���Ҫ�õ�3mol��L-1������100mL�����Ƹ�������Һʱ�������õ��ձ�������������ͷ�ι��⣬����Ҫ��������_________
��4������ܼ�������X��Ŀ����_____������X����ѡ�����������е�_____��
A���������� B��ϡ���� C����ˮ D������ͭ
��5������Һ�н�������Ũ�ȵ���1��10-5 mol��L-1ʱ����Ϊ�ý������ӳ�����ȫ����Ksp[Fe(OH)2]= _____��