��Ŀ����
�Ա���ۣ���Ҫ�ɷ�ΪBaCO3������Ca2+��Fe2+��Fe3+��Mg2+�ȣ��Ʊ�BaCl2��2H2O���������£� 
��1������������Ҫ��Ӧ�����ӷ���ʽΪ ��
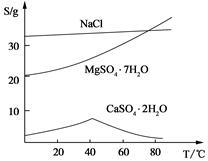
��2������C����Ҫ�ɷ���Ca(OH)2�� ����ͼ��֪��Ϊ�˸��õ�ʹCa2+��������Ӧ��ȡ�Ĵ�ʩΪ ��

��3����BaSO4�������ⶨ��Ʒ���ȵIJ���Ϊ��
����1��ȷ��ȡ0.4��0.6 g BaCl2��2H2O����������100 ml ˮ��3 ml 2 mol��L-1��HCl��Һ�����ܽ⡣
����2���߽��裬����μ���0.1 mol��L-1 H2SO4��Һ��
����3����BaSO4������ ��ȷ������ȫ������
����4�����ˣ���0.01 mol��L-1��ϡH2SO4ϴ�ӳ���3~4�Σ�ֱ��ϴ��Һ�в���Cl��Ϊֹ��
����5�����۵��ij�����ֽ������ �У�����ɡ�̿�����һ�����800�����������ء���������BaCl2��2H2O��Ba2+�ĺ�����
�ٲ���3��ȱ�IJ���Ϊ ��
��������1��������Ʒ���٣����ڲ���4ϴ��ʱ������ɵ�Ӱ��Ϊ ��
�۲���5���ô�����������Ϊ ����ֽ�һ�ʱ����Ҫ���㣬����BaSO4�ױ�������̿��ԭ����BaS���÷�Ӧ�Ļ�ѧ����ʽΪ ��
����ͬѧ��Ϊ��K2CrO4����H2SO4��������Ч�����ã���˵��ԭ�� ��
[��֪��Ksp(BaSO4)=1.1��10-10 Ksp(BaCrO4)=1.2��10-10]
��1��H2O2+ 2Fe2++2H+ =2Fe3+ +2H2O
��2��Mg(OH)2 ����¶�
��3�������ϲ���Һ�м���1~2��0.1mol/LH2SO4��Һ
�ڳ�ȡ�������٣����������٣�ϴ����ɵ���ʧ�ʹ�
������ BaSO4 + 4C 4CO + BaS��BaSO4 + 2C
4CO + BaS��BaSO4 + 2C 2CO2 + BaS
2CO2 + BaS
��BaCrO4��Ħ����������BaSO4
�����������������Ϊ���������⣬����Ҫ�ɷ�ΪBaCO3����ۣ�������ȥ����Ca2+��Fe2+��Fe3+��Mg2+�������Ʊ�BaCl2��2H2O�����̡���2������C֮ǰ�ѳ�ȥ�������Ըò��dz�ȥʣ�������Ca2+��Mg2+��������Ӧ���������ͼ������Ca(OH)2�ܽ�����¶����߶����ͣ��ʸ�����������Ca2+��������3���ٲ���3��Ȼ��ȷ�������Ƿ���ȫ�IJ�����Ӧ���Ǿ��ã������ϲ���Һ�м���1~2��ԭH2SO4��Һ���������ֳ�������Ba2+������ȫ���ڳ�ȡ�������٣����������٣�ϴ����ɵ���ʧ�ʹ۹�������һ�㶼�������У����������ʵ�Ksp������ͬ����BaCrO4��Ħ����������BaSO4��ʵ�������ɵij��������������С��
���㣺���������⣬�������ʵķ��롢���ӡ����顢ʵ������Ȼ���ʵ��������й����⡣

������ʵ�������Ԥ��ʵ��Ŀ�Ļ�����ʵ����ۣ���ȷ����̣�����������
| ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� | �ж� |
| �� | ij�����������ᣬ������ʹ����ʯ��ˮ����ǵ���ɫ��ζ���� | ˵���ü����� | |
| �� | ��ij��Һ�м������ᣬ�����������������м���BaCl2��Һ�а�ɫ���������� | ֤������Һ���� SO42�� | |
| �� | �������Һ�м���һ������ϡ������ȣ��ټ���һ����������������ͭ���ȡ� | ֤������ˮ����������� | |
| �� |  ��Ũ����170�湲�ȣ��Ƶõ�����ͨ������ ��Ũ����170�湲�ȣ��Ƶõ�����ͨ������ ��Һ ��Һ | �����Ƶ������Ƿ�Ϊ��ϩ | |
ijͬѧ�ӱ������˽�����и��������Բ����κ�̼���Σ�������ʵ����֤��һ��ʵ���������κ�������һ���������ϵ�֪�����ᣨH2C2O4��������ǿ�ڴ���Ķ�Ԫ�л��ᣬ����һ�ֻ�ԭ�Խ�ǿ�����ʣ���2KMnO4+5H2C2O4+3H2SO4=2MnSO4+K2SO4+10CO2��+8H2O������ƣ�CaC2O4��������ˮ�ʹ��ᣬ������ǿ�CaC2O4+2H+= H2C2O4+Ca2+��
��1�����ʵ�鷽����֤�����к��в����κ�̼���Σ��������ʵ�鲽�衢Ԥ������ͽ��ۡ�
��ѡ�Լ���1 mol��L��1 H2SO4��1 mol��L��1 HCl��0.1 mol��L��1 NaOH��1 mol��L��1 CaCl2��0.01 mol��L��1 KMnO4������ʯ��ˮ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1����������ĥ���ݡ����˵õ����������Һ�� | |
| ����2��������Һ�������ԣ��μ�����CaCl2��Һ�� | ���ְ�ɫ������˵�������п��ܺ��в����κ�̼���Ρ� |
| ����3��ȡ����2�ij������Թ��У� | |
| ����4�� | |
��2��Ҫȷ�ⶨ�����в����κ�������ش��й����⣺
�� ������ȡm g������Ʒ�������в�����ת��ΪCaC2O4��������������������ܽ����Һת��_________�м�ˮ���Ƴ�100mL��Һ��ÿ����_____________��ȡ25.00mL����Һ����0.0100mol��L��1 KMnO4����Һ�ζ���ƽ�����ı���ҺV mL��
�� ���㣺�����в����Σ���C2O42���ƣ�����������Ϊ___________________����ֻ��ʽ�����㡣C2O42���Ļ�ѧʽ��Ϊ88��
�� ���ۣ��ñ�KMnO4��Һֱ�ӵζ�������ĥ�����ݡ����˵õ�����Һ���������ƫ�ߣ�ԭ���� ��
�屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ���й��������£�
| | �� | �� | �屽 |
| �ܶ�/g��cm-3 | 0. 88 | 3. 10 | 1. 50 |
| �е㣯�� | 80 | 59 | 156 |
| ˮ���ܽ�� | �� | �� | �� |
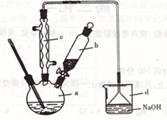
�����кϳɲ���ش����⣺
��1����a�м���15mL��ˮ����������м����b��С�ļ���4.0mLҺ�塣��a�е��˼���Һ�壬�а�������������Ϊ������ ���塣�����μ���Һ����ꡣװ��d��������_________________________ ��
��2��Һ�����������в�������ᴿ��
����a�м���l0mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����Һ������10mLˮ��8mLl0%��NaOH��Һ��l0mLˮϴ�ӡ�NaOH��Һϴ�ӵ�������
��
����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����ˡ������Ȼ��Ƶ�Ŀ����
��
��3�������Ϸ���������屽�л����е���Ҫ����Ϊ ��Ҫ��һ���ᴿ���в����б������ ��������ȷѡ��ǰ����ĸ����
A���ؽᾧ B������ C������ D����ȡ
��4���ڸ�ʵ���У�a���ݻ����ʺϵ��� ��������ȷѡ��ǰ����ĸ����
A��25mL B��50mL C��250mL D��500mL
��������ȿ�����Ũ�������ֿ����ù����ʯ�Ҹ������
| A��SO2 | B��NH3 | C��Cl2 | D��H2 |