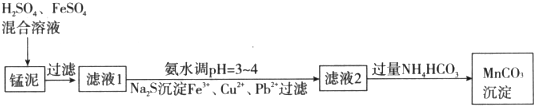
��Ŀ����
20��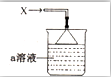 ��ͼ��ʾ���ձ���ʢ�е�a��Һ�����������ӣ�������PH=1����������һ��������Һ��������X��������õ�һ��w��Һ��W��Һ��ɫ���������������ӣ�����һ�����Ӻ���18�����ӣ��������Ӹ�����10�����ӣ�����˵����ȷ���ǣ�������
��ͼ��ʾ���ձ���ʢ�е�a��Һ�����������ӣ�������PH=1����������һ��������Һ��������X��������õ�һ��w��Һ��W��Һ��ɫ���������������ӣ�����һ�����Ӻ���18�����ӣ��������Ӹ�����10�����ӣ�����˵����ȷ���ǣ�������| A�� | ����X��Һ����ԭ������Է��������ϴ� | |
| B�� | W��Һ����������������������γɵ����ӻ����������� | |
| C�� | ��a��Һ����һ����������X����ҺpH=7����ʱ��Һ����4������ | |
| D�� | a��Һ����ˮ�������c��H+��=10-13mol•L-1 |
���� �ձ���ʢ�е�a��Һ�����������ӣ�������PH=1��˵����Һ�����ԣ���������һ��������Һ��������X��������õ�һ��w��Һ���ƶ�XΪNH3�����ɵ�WΪNH4Cl��W��Һ��ɫ���������������ӣ��ֱ�ΪCl-��NH4+��OH-��H+������һ�����Ӻ���18������ΪCl-���������Ӹ�����10������ΪNH4+��OH-��
A���������Ӽ������������Ĵ���ʹ�÷��Ӽ���������ǿ��ʹ�ð�����Һ����
B��W��Һ��ɫ���������������ӣ��ֱ�ΪCl-��NH4+��OH-��H+������������ɵ����ӻ�����ΪNH4Cl��
C����a��Һ����һ����������X����ҺpH=7��NH3��HCl������Ӧ���ɵ��Ȼ����Һ�����ԣ������Թ�������Һ������ΪNH4Cl��NH3•H2O��
D��aΪHCl��Һ������Ũ��Ϊ0.1mol/L����ˮ�������������Ũ�ȵ���ˮ���������������Ũ�ȣ��������ӻ���������õ���
��� �⣺�ձ���ʢ�е�a��Һ�����������ӣ�������PH=1��˵����Һ�����ԣ���������һ��������Һ��������X��������õ�һ��w��Һ���ƶ�XΪNH3�����ɵ�WΪNH4Cl��W��Һ��ɫ���������������ӣ��ֱ�ΪCl-��NH4+��OH-��H+������һ�����Ӻ���18������ΪCl-���������Ӹ�����10������ΪNH4+��OH-��
A���������Ӽ������������Ĵ���ʹ�÷��Ӽ���������ǿ��ʹ�ð�����Һ��������Է��������أ���A����
B��W��Һ��ɫ���������������ӣ��ֱ�ΪCl-��NH4+��OH-��H+������������ɵ����ӻ�����ΪHCl��NH4Cl��NH3•H2O��H2O������ֻ��NH4Clһ�����ӻ������B����
C����ҺpH=7��NH3��HCl������Ӧ���ɵ��Ȼ����Һ�����ԣ������Թ�������Һ������ΪNH4Cl��NH3•H2O����C����
D��aΪHCl��Һ������Ũ��Ϊ0.1mol/L����ˮ�������������Ũ���������ӻ���������õ���c��OH-��ˮ=c��H+��ˮ=$\frac{1{0}^{-14}}{0.1}$=10-13mol/L����D��ȷ��
��ѡD��
���� ���⿼���˵������Һ���������ʵķ����жϣ������������ʵ�����Ӧ�ã������Ե���Һ�������ռ�������Ϊ�����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| A�� | �����е�C��H��O�ĸ�����Ϊ1��2��3 | B�� | ������C��H������Ϊ1��2 | ||
| C�� | ���л������Է�������Ϊ14 | D�� | �÷����п϶�������Ԫ�� |
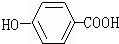 ת��Ϊ
ת��Ϊ ������Լ��ǣ�������
������Լ��ǣ�������| A�� | Na | B�� | NaHCO3 | C�� | Na2CO3 | D�� | NaOH |
| A�� | c��Na+����c��A-����c��H+����c��OH-�� | B�� | c��A-��+c��HA��=0.05mol•L-1 | ||
| C�� | c��Na+����c��A-����c��HA����c��OH-�� | D�� | c��HA��+c��H+��=c��A-��+c��OH-�� |
| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
| A�� | ��ʪ���pH��ֽ�ⶨ��Һ��pH���ⶨ���ƫС | |
| B�� | ʯ���̪�����ָʾ�����������к͵ζ���ָʾ�� | |
| C�� | ������ƽ����ҩƷʱ����Ӧ������ֽ���� | |
| D�� | �ζ����Լ�����ƿ��ʹ��ǰ�����Ƿ�©ˮ |
| A�� | IA�壬���ʵ��۵����� | |
| B�� | ��A�壬����̬�⻯�ﻹԭ������ǿ | |
| C�� | VA���⻯��ķе������� | |
| D�� | ��A������������Ӧˮ�������������ǿ |
| pH | 2 | 4 | 6 | 6.5 | 8 | 13.5 | 14 |
| ��ʴ���� | �Ͽ� | �� | abc �Ͽ� | ||||
| ��Ҫ���� | Fe2+ | Fe3O4 | Fe2O3 | FeO2- | |||
| A�� | ��pH��4ʱ��̼����Ҫ�������ⸯʴ | |
| B�� | ��pH��6ʱ��̼����Ҫ����������ʴ | |
| C�� | ��pH��14ʱ��������ӦΪO2+4H++4e��2H2O | |
| D�� | ����г�������ļ�����Һ�У�̼�ָ�ʴ���ʻ���� |