��Ŀ����
����Ŀ��Ni��Fe�ڹ�ҵ���������Ͳ��������й㷺��Ӧ�ã������ơ����˽𡱣���ѧ�ɷ���FeS2�������������徧ϵ������������Ϊ�ǻ������ʡ��¶����ߺ��û��á��ڿ����������������������Ͷ���������Ҫ���ڽӴ���������ش��������⣺
��1����FeS2��ϡ��������Ӧ�õ�H2S2��H2S2�����У����ۼ���������___________________��FeS2�����õ�SO2����SO2�����е�Sԭ�ӵ��ӻ����������______________________��
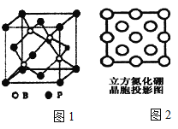
��2��FeS2�ľ����е�Fe2+���ӵ����з�ʽ����ͼ��ʾ��

��ÿ��Fe2+��Χ����ĵȾ����S22-������________����
����֪FeS2�ľ���������a0=54nm�������ܶ�Ϊ_____________gcm-3��(��ʽ�����㣬�����ӵ�����Ϊ6.02��1023)��
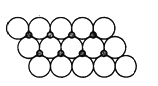
��3��NiO����ṹ��NaCl�������ƣ��侧�����ⳤΪacm����þ����о�����������������Ӻ˼�ľ���Ϊ___________(�ú���a�Ĵ���ʽ��ʾ)����һ���¶��£�NiO��������Է��ط�ɢ���γɡ������Ӳ㡱(����ͼ)��������Ϊ�����������µ������У�������������У���ʽ������ÿƽ��������Ϸ�ɢ�ĸþ��������Ϊ________g(�����ӵİ뾶Ϊ1.40��10-10m��![]() =1.732)��
=1.732)��
���𰸡���1�����Լ��ͷǼ��Լ��γɦļ� sp2
��2����6 ����=![]() =
=![]() =5.06
=5.06
��3��![]() ��
��![]() 1.83��10-3(g)
1.83��10-3(g)
��������
�����������1��H2S2�����У�����H-S��Ϊ���Թ��ۼ���S-S��Ϊ�Ǽ��Թ��ۼ�����SO2�����е�Sԭ�ӵļ۲���Ӷ���Ϊ![]() =3������Sԭ�ӵ��ӻ��������Ϊsp2�ӻ���
=3������Sԭ�ӵ��ӻ��������Ϊsp2�ӻ���
��2����ÿ��Fe2+��Χ����ĵȾ����S22-���Ӵ��������ģ�����6����
��һ�������ں���Fe2+��ĿΪ8��![]() +6��
+6��![]() =4������S2-��ĿΪ12��
=4������S2-��ĿΪ12��![]() +1=4��һ������������m=
+1=4��һ������������m=![]() ��һ���������V=a03�����ܶȦ�=
��һ���������V=a03�����ܶȦ�=![]() =
=![]() =5.06gcm-3��
=5.06gcm-3��
��3�������Ȼ��ƵĽṹ֪�������Ӻ����ڵ�������֮��ľ���Ϊ![]() ��������������������Ӻ˼�ľ����Ǿ�������������Ӻ������Ӿ����
��������������������Ӻ˼�ľ����Ǿ�������������Ӻ������Ӿ����![]() ���������������
���������������![]() acm������ͼƬ֪��ÿ����������ռ�����=2��1.40��10-10m��2��1.40��10-10m��sin60�㣬��ÿƽ�����е�����������=
acm������ͼƬ֪��ÿ����������ռ�����=2��1.40��10-10m��2��1.40��10-10m��sin60�㣬��ÿƽ�����е�����������=![]() ��ÿ��������������=
��ÿ��������������=![]() g������ÿƽ�����е������������T
g������ÿƽ�����е������������T![]() =1.83��10-3��
=1.83��10-3��