��Ŀ����
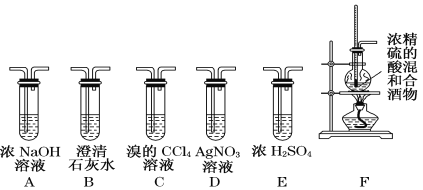
����Ŀ���±���A��B��C��D��E�����л�����й���Ϣ
�ش��������⣺
��1��A��E�У�����������___________ ������ĸ����
��2��A��ʹ������Ȼ�̼��Һ��ɫ��������Ӧ�Ļ�ѧ����ʽΪ __________
��3��C��E���ɵ����ڼ��ԣ�����������Һ��������ˮ��Ļ�ѧ����ʽΪ _______
��4���л���B���е�������________________������ţ���
����ɫ��ζ��Һ�壻���ж�����������ˮ�����ܶȱ�ˮ������ʹ����KMnO4��Һ����ˮ��ɫ
��5��E��ˮ��Һ��ʹ��ɫʯ����Һ��죬˵��E��ˮ��Һ�����ԣ������һ��ʵ�����Ƚ�E��̼�����Ե�ǿ�������ʵ���ԭ���� _____���û�ѧ����ʽ˵������
���𰸡�AB CH2=CH2+Br2��BrCH2CH2Br CH3COOCH2CH3 + NaOH �� CH3COONa + CH2CH3OH �ڢ� CH3COOH+NaHCO3=CH3COONa+H2O+CO2��
��������
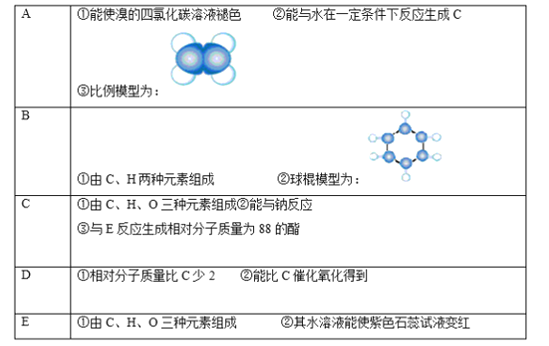
A��ʹ���CCl4��Һ��ɫ��˵������̼̼˫������������ϱ���ģ��֪��AΪ��ϩ��A����ˮ��һ�������·�Ӧ����C����CΪ�Ҵ�������B��C��H����Ԫ����ɼ������ģ�Ϳ�֪��BΪ����D����Է���������C��2������C�������ɣ�����DΪ��ȩ����E�����Ԫ�ؼ����Ҵ���Ӧ������Է�������Ϊ88��������ˮ��Һ��ʹ��ɫʯ����Һ����֪��EΪCH3COOH���ݴ˴��⡣
�ɷ�����֪��AΪ��ϩ��BΪ����CΪ�Ҵ���DΪ��ȩ��EΪ���ᡣ
��1��������������5���л�����ֻ����ϩ�ͱ�����������A��E�У����������ǣ�A��B���ʴ�Ϊ��AB��
��2����ϩ��������Ȼ�̼��Һ�����ӳɷ�Ӧ����1��2���������飬��ѧ����ʽΪ��CH2=CH2+Br2��CH2BrCH2Br���ʴ�Ϊ��CH2=CH2+Br2��CH2BrCH2Br��
��3��CΪ�Ҵ���EΪ���ᣬ�������Ҵ���Ӧ����������������������������������Һ�����·���ˮ�ⷴӦ���������ƺ��Ҵ�����Ӧ����ʽΪ��CH3COOCH2CH3+NaOH��CH3COONa+CH2CH3OH���ʴ�Ϊ��CH3COOCH2CH3+NaOH��CH3COONa+CH2CH3OH��
��4��BΪ���������±�����ɫ����������ζ��Һ�壬�ܶȱ�ˮС��������ˮ���ж����������к��в����ͼ���һ�������¿��������������ӳɷ�Ӧ������̼̼˫����������������ʹ���Ը��������Һ����ˮ��ɫ���ʢڢ���ȷ���ʴ�Ϊ���ڢۡ�
��5������ǿ����ȡ�����ԭ��֤����������Դ���̼�ᣬ�����NaHCO3��Ӧ���ɶ�����̼�����֤����������Դ���̼�ᣬ��Ӧ����ʽΪ��CH3COOH+NaHCO3��CH3COONa+H2O+CO2�����ʴ�Ϊ��CH3COOH+NaHCO3��CH3COONa+H2O+CO2����

����Ŀ������ʵ���е���ɫ�仯����������ԭ��Ӧ�ص���
A | B | C | D | |
ʵ�� | ������ˮ����Na2S ��Һ�� | �Ҵ�����K2Cr2O7������Һ�� | ����FeCl3��Һ�����ˮ�� | �������KMnO4 ������Һ�� |
���� | ������ɫ���� | ��Һ�ɳ�ɫ��Ϊ��ɫ | Һ���Ϊ���ɫ�ҳ����� | ������ɫ���壬��Һ�Ϻ�ɫ��ȥ |
A. AB. BC. CD. D