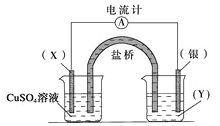
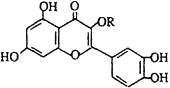
��Ŀ����
����Ŀ��A��B��C��DΪ����ԭ��������������Ķ�����Ԫ�أ�����B��C��Dλ��ͬһ���ڣ�A��B�γɵķ��Ӿ��жԳƵ���������ṹ��C�ĵ��ʷ�����NO+(����������)�ĵȵ����壬D��Ԫ�����ڱ��е縺������Ԫ�ء������������ش��������⣺
��Bԭ�ӵĺ�������Ų�ʽ��______________��
��д������A��B���γɵķ��ӵĵ���ʽ��____________��
��NO���Ľṹ�п��ܺ�____����������___��������
��д��B��C���⻯������幹�ͷֱ���________��_________��
���������ʾʽд��D���⻯����Һ�д��ڵ��������______________��
���𰸡�1s22s22p2  1 2 �������� ���� F�DH��F��F�DH��O��O�DH��F��O�DH��O
1 2 �������� ���� F�DH��F��F�DH��O��O�DH��F��O�DH��O
��������
A��B��C��DΪ����ԭ��������������Ķ�����Ԫ�أ�����B��C��Dλ��ͬһ���ڣ�A��B�γɵķ��Ӿ��жԳƵ���������ṹ����A��HԪ�أ�B��CԪ�أ�C�ĵ��ʷ�����NO+(����������)�ĵȵ����壬NO+(����������)�ĵȵ�������N2����C��NԪ�أ�D��Ԫ�����ڱ��е縺������Ԫ�أ���D��FԪ�أ��ݴ˷������
��������������֪A��HԪ�أ�B��CԪ�أ�C��NԪ�أ�D����FԪ�ء�
(1)B��CԪ�أ�Cԭ�Ӻ�����6�����ӣ���ԭ�ӵĺ�������Ų�ʽ��1s22s22p2��
(2)A��B����Ԫ�ص�ԭ�����γɵľ�����������ṹ�ķ�����CH4���÷�����Cԭ�ӵ��������4�����ӣ���4��Hԭ�ӵĵ��ӷֱ��γ�1�Թ��õ��Ӷԣ��Ӷ�ʹÿ��ԭ�Ӷ��ﵽ�ȶ��ṹ������CH4�ĵ���ʽ�ǣ� ��
��
��NO����N2��Ϊ�ȵ����壬�ȵ�����Ľṹ���ƣ�N2������2��Nԭ���γ��������ۼ������Կ���֪NO���ṹ��Ҳ���������ۼ������к�1����������2��������
(4) B��C��C��N�����ǵ��⻯����⻯��ֱ���CH4��NH3������CH4�����幹�������������Σ�NH3�������Σ�
(5) D���⻯����HF��HF����Һ�л��γ����������HF����֮���γɵ����F�DH��F��ˮ����֮������O�DH��O��HF������ˮ����֮���γɵ����F�DH��O��O�DH��F�����������͡�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
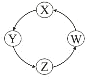
Сѧ��10����Ӧ����ϵ�д�����Ŀ�����и��������У��������ͼʾ������һ�������£�һ��ת����ϵ������У� ��
��� | X | Y | Z | W |
|
�� | Si | Na2SiO3 | H2SiO3 | SiO2 | |
�� | Na | NaOH | Na2CO3 | NaCl | |
�� | Cl2 | Ca(ClO)2 | HClO | HCl | |
�� | Fe | FeCl3 | FeCl2 | Fe(OH)2 |
A.�٢ڢ�B.�٢�C.�ڢ�D.�٢�