��Ŀ����
����Ŀ�����к����ۡ�NaCl��Na2SO4��CaCl2�Ļ����Һ��ѡ���ʵ����Լ��ͷ��������ᴿ��NaCl���塣��Ӧ��ʵ��������£�
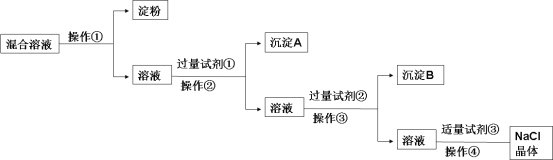
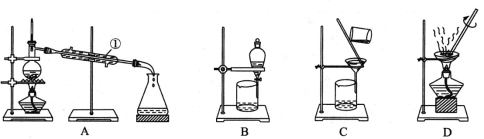
��1�������������ý��岻��ͨ�� ���ѵ��۽������Һ���з��롣
��2��д������ʵ������������Լ��Ļ�ѧʽ��
�Լ���___________________���Լ���_____________________��
��3���ж��Լ����ѹ����IJ����ǣ�__________________��
��4�������Լ��������ķ����ǣ�__________________��
��5���Լ��������ã��û�ѧ����ʽ��ʾ����__________________��
���𰸡���1����Ĥ��2�֣���2��BaCl2��2�֣���HCl(2�֣�
��3�����ã����ϲ���Һ�еμ������Ȼ�����Һ��û�а�ɫ����������˵���Ȼ�����Һ�ѹ�������2�֣�
��4���μ�ϡ��������Һ�����ԣ���μ�ϡ��������ҺpHֵ��7��μ�ϡ��������Һ���ٲ������ݣ���2�֣�
��5��BaCl2+Na2CO3��2NaCl+BaCO3����CaCl2+Na2CO3��2NaCl+CaCO3��
��������
������������岻������Ĥ��������Ϊ��������ȥNa2SO4��CaCl2���ɷֱ����BaCl2��Na2CO3����ȥ�����к��е�Ca2+��SO42-���������ʵķ������������BaCl2��ȥ����������ӣ��ټ������Na2CO3��ȥ�������ӣ������Լ���ΪBaCl2��������Ϊ���ˣ�����AΪ���ᱵ���Լ���ΪNa2CO3��������Ϊ���ˣ�����BΪ̼��ƺ�̼�ᱵ���Լ���Ϊ���ᣬ��������ɳ�ȥ������Na2CO3����������ᾧ�ɵõ�NaCl���塣
��1�������������ý��岻��ͨ����Ĥ���ѵ��۽������Һ���з��룻
��2�������Ϸ�����֪�Լ���ΪBaCl2���Լ���ΪHCl��
��3���ж��Լ����ѹ����ķ����Ǿ��ã����ϲ���Һ�еμ������Ȼ�����Һ��û�а�ɫ����������˵���Ȼ�����Һ�ѹ�����
��4������������dz�ȥ̼���ƣ����Կ����Լ��������ķ������μ�ϡ��������Һ�����ԣ���μ�ϡ��������ҺpHֵ��7��μ�ϡ��������Һ���ٲ������ݣ���
��5���Լ���ΪNa2CO3�������Լ����������dz�ȥ��Һ�и����Ӻ����ı����ӣ���Ӧ�Ļ�ѧ����ʽΪBaCl2 +Na2CO3 ��2NaCl+BaCO3����CaCl2 +Na2CO3 ��2NaCl+CaCO3��